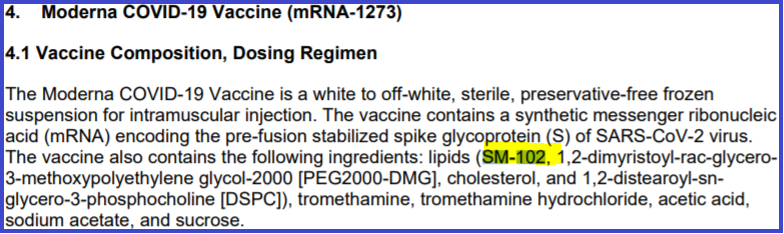
Is a radio host correct in saying the Material Safety Data Sheet for one part of an mRNA vaccine proves there is a poison in the Moderna COVID-19 vaccine? No, that's not true. Chemicals in which lipids like SM-102 are maintained before use are removed in the manufacturing process, the way the lye used to preserve cod is removed from lutefisk before it is cooked and eaten. Lipids are a fat that form the building blocks of human cells and to get them to labs and factories, they have to be held in a preservative solution. Federal regulations require information sheets on each version of many materials used in laboratories and factories and include the solution in which they are maintained until prepared for addition to the final product. The data sheet referred to in the radio talk show host's claim is for the research grade of the lipid called SM-102, not the manufacturing grade material.
The claim appeared in an article (archived here) where it was published by Hal Turner Radio Show on May 17, 2021, under the title "Connecticut Publishes Moderna COVID Vax Ingredients: DEADLY POISON 'SM-102 - Not for Human or Veterinary Use'". It continued:
This appears to be what they are injecting into YOUR arm when you take the Moderna COVID Vax. You are APPARENTLY being POISONED!
This is what the article looked like on HalTurnerRadioShow.com at the time of writing:
(Source: HalTurnerRadioShow.com screengrab taken Tue May 18 20:02:53 UTC 2021)
The writer of the "SM-102" article appears to be ignorant of the basics of chemical lab and manufacturing processes and of the Materials Safety Data Sheet (MSDS) system, which can be made to sound scary by insisting the medium in which lipids are sent to labs is then dumped into syringes and injected into arms. This would be akin to shouting that lectin-containing beans are being used to poison consumers or that apple growers are selling a product "Not for human use." The process of soaking red kidney beans removes the lectin and people process apples by removing the seeds, which contain cyanide.
The "SM-102 poison" claim also relies on the wrong MSDS sheet, said Jesse Erasmus, a Ph.D. virologist working as a medical school professor and as a postdoctoral fellow in the University of Washington lab of Deborah Fuller, who led studies of the mRNA vaccines' effects on animals, among the safety tests undertaken before the vaccines were approved by the FDA to fight the pandemic. The lab confirmed a central hope for mRNA vaccines: that they would work well in the immune systems of aging humans, which they do. In a May 18, 2021 email, to Lead Stories, Erasmus said the Turner claim is based on several false assumptions:
I can just say that the MSDS sheet that they are referring to is not that of the actual ingredient source used to manufacture the vaccine. These ingredients are manufactured at different quality grades, including research grade and good manufacturing practices (GMP) grade. The MSDS sheet they refer to is one example of a research grade material and that explains why they have a statement saying that it is not for use in humans. Additionally, the lipid is maintained in a buffer containing chloroform which is a highly volatile organic solvent and the MSDS sheet takes that into account. For cGMP manufacturing, Moderna would need to source a GMP-grade SM102 lipid and any organic solvent used (not sure if it would be chloroform in their case) would be removed during the manufacturing process.
The writer of the "SM-102" article had also apparently not researched the FDA's approval of the Moderna vaccine. The documents HalTurnerRadio.com found in Connecticut don't constitute a scoop, since the December, 2020 FDA announcement notes the use of the lipid as follows:
(SOURCE: FDA.gov screenshot (highlighting added by Lead Stories) taken Tue May 18 at 21:36:54 UTC 2021)
Lead Stories has reached out to the Food and Drug Administration and to Moderna for additional information and will update this fact check, as appropriate, when they reply.