STORY UPDATED: check for updates below.
Did HUD Secretary Dr. Ben Carson announce that he is treating his COVID-19 with oleander? No, he didn't "announce" it, but he did confirm to Lead Stories that oleander, an extract for the leaves of oleander trees, is one of the "therapeutics" he is taking. The secretary of Housing and Urban Development, who is also a retired neurosurgeon, confirmed to Lead Stories in a phone call on November 16, 2020, that he believes oleander is helping his recovery from COVID-19. Dr. Carson said he is "not advocating just put it out there and have people start taking it in a therapeutic way," but he does want the Food and Drug Administration to approve clinical trials as a potential therapy for COVID-19 patients.
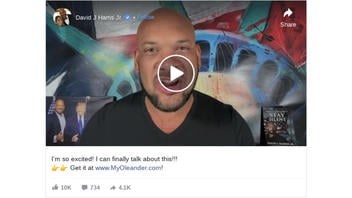
The oleander claim originated in a Facebook post (archived here) on the page of homeopath David Harris on November 11, 2020, under the title "I'm so excited! I can finally talk about this!!!" Harris says in his video:
"I can 100 percent confirm that this, that Dr. Ben Carson is talking about a powerful therapeutic, is this product right here. You can get it at MyOleander.com."
This is what the post looked like on Facebook at the time of writing:
(Source: Facebook screenshot taken on Wed Nov 11 22:01:52 2020 UTC)
Here's a transcript of the relevant part of Harris' video.
Some of you have heard me talk about a therapeutic, an all-natural remedy, an all-natural extract that absolutely works. I mean, the president knows about it, Ben Carson knows about it, they've been briefed on it. They were trying to get it past the FDA. The FDA was like, 'Nah, we're not going to do that' because I don't think they want a cure. I don't think they want something actually works.
Well, my dad got COVID. I've had several friends that have gotten COVID, tested positive, were having horrible, horrible symptoms, like 103, 104 temperature, shakes, couldn't sleep at night. They started taking what Dr. Ben Carson just announced that he took, this therapeutic. I can 100 percent confirm that this, that Dr. Ben Carson is talking about, a powerful therapeutic is this product right here.
You can get it at MyOleander.com. I am friends with the CEO of that company. I know that it works and it's something that if you, at all, have any symptoms, or you want to make sure that your loved ones are taken care of, get this now. My Oleander.com. Go get yourself a bottle, make sure you've got it for your friends, for your family. Make sure that you're prepared. I know that it works. I know it does 100 percent. it's worked for every single person, every single friend of mine, and even my dad, that's tried it. They've all recovered and gotten well."
Carson on Monday, November 9, 2020, published on Facebook a message thanking well-wishers:
Thankfully I have access to a very powerful therapeutic, so I was only sick for a very short period of time and I am already back to working, albeit virtually.
Carson's Facebook post makes no mention of oleander.
We are so touched by the outpouring of concern and prayers after the news came out that I had tested positive for...
Posted by Dr. Ben & Candy Carson on Monday, November 9, 2020
Lead Stories reached out to Carson before publishing this debunk article. When he called us five days later, November 16, he said he was "recuperating nicely," and he confirmed that oleander was the therapeutic he was taking for his illness.
Yes, that's what it was.
Dr. Carson said that while he is not suggesting others should take oleander, he does want the FDA to approve clinical trials:
I believe in it and, you know, urging the FDA to go ahead and do some trials. I am not advocating just put it out there and have people start taking it in a therapeutic way. I'm saying you've got something that has a lot of evidence, both laboratory and anecdotal evidence, and you've got a lot coming down with the disease. So, why wouldn't you do some clinical trials and verify one way or another. This is something that could be very helpful and could save a lot of lives.
He added that too little is known now about how much oleander is safe and effective to take:
All of that is being guessed at and a lot of the anecdotal stories, you know, it's being taken four times a day, sublingually. But do we really know what the optimal dose and timing is? No, and that's one of the reasons that you do the clinical trials.
Carson is one of 27 members of the President's Coronavirus Task Force, to which he was appointed on March 1, 2020. Carson's chief of staff announced on November 9, 2020, that the 69-year-old retired surgeon had tested positive for COVID-19.
An FDA press spokesperson emailed Lead Stories to say the drug safety agency has rejected attempts to make the active ingredient, oleandrin, a health product. Courtney Rhodes wrote that the FDA does not know what specific product Secretary Carson is using and cannot comment on that product:
FDA has not approved any oleander product for the diagnosis, cure, mitigation, treatment or prevention of any disease, including COVID-19.
Rhodes wrote that the FDA in 1998 objected to a manufacturer's proposal to add a Nerium oleander extract to health products:
In our response letter, FDA stated that "N. oleander is well known to be a poisonous plant. All parts of the oleander plant are poisonous to man and animals and serious adverse effects are associated with ingestion, inhalation, and contact of mucus membranes with oleander or oleander extracts."
In August 2020, FDA again objected to a manufacturer's proposal to make oleander an approved health ingredient, said Rhodes:
The agency concluded that Oleandrin is excluded from the definition of dietary supplement and therefore may not be marketed as or in a dietary supplement. The FDA also expressed significant concerns about the evidence included in the notification to establish that Oleandrin will reasonably be expected to be safe under the conditions of use described in the notification.
Rhodes said the FDA "strongly advises consumers to avoid consuming or otherwise using any product containing oleandrin, a cardiac glycoside, or any other oleander extracts. This includes any product that misleadingly purports to cure, mitigate, treat or prevent any disease, including COVID-19.
In a November 13, 2020 email to Lead Stories, a Department of Defense spokesman said its researchers called off testing of oleandrin:
U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID) assessed the product in May in a neutralization test. The neutralization test, performed in a culture dish, determines if a product can stop viral infection in cells. Following multiple iterations of the test, their results were inconclusive if the product had anti-viral activity against SARS-CoV-2. USAMRIID discontinued testing and focused efforts on evaluating other therapeutics and compounds to treat and prevent SARS-CoV-2.
The American Herbal Products Association in August, 2020 issued a statement discouraging any medical use of any part of the oleander plant, including for treatment of COVID-19.
Updates:
-
2020-11-16T19:25:48Z 2020-11-16T19:25:48Z Dr. Carson confirms to Lead Stories he is using oleander as a therapy for his COVID-19. FDA and Department of Defense respond regarding their findings that oleandrin is not recommended.