Does this list of reports from the federal Vaccine Adverse Event Reporting System (VAERS) prove COVID-19 vaccines are causing sudden deaths in a surprising number of young and middle-aged people? No, that's not true: The individual cases in the VAERS system - as the VAERS builders clearly state, have not been verified. Anyone can file a report to the VAERS system and the database does not indicate which, if any, have been confirmed as vaccine-caused reactions. Doctors are required by law to report all adverse events that occur within a certain period of time after vaccination. A Lead Stories spot-check of 10 random cases of the 28 listed revealed that many of those listed had pre-existing serious health conditions and were residents of long-term care facilities, where mortality rates are much higher than in the population at large.
The list of 28 VAERS reports with ID numbers appeared in a post (archived here) where it was published by the Facebook page "Learn the Risk" on February 9, 2021. The post contains no introduction although it finishes with instructions for navigating the search features of the VAERS database. Below is a sample of the presentation of three cases from the top of the list:
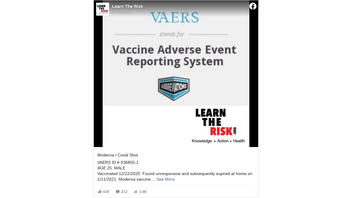
VAERS ID # 936805-1.AGE 25. MALEVaccinated 12/22/2020. Found unresponsive and subsequently expired at home on 1/11/2021. Moderna vaccine.*VAERS ID # 943397-1AGE 28. MALEVaccinated 12/23/2020. Died 1/14/2021. Patient was found unresponsive at work in the hospital. Patient pupils were fixed and dilated. Pfizer vaccine.*VAERS ID # 939050-1AGE 32. FEMALEVaccinated 12/28/2020. Died on 1/4/21 at 7:20am. Moderna vaccine.
This is what the post looked like on Facebook at the time of writing:
(Source: Facebook screenshot taken on Fri Feb 12 22:11:49 2021 UTC)
Given its posting on a Facebook page dedicated to deterring people from taking vaccines, it is clear that the intention of publishing this list is to deter people from taking the COVID-19 vaccine. The "Learn the Risk" mission statement, found at Learntherisk.org leaves little question of the intent of posting a list of vaccine reaction reports:
Learn The Risk is a US-based, non-profit organization and a powerful force for educating people WORLDWIDE on the dangers of pharmaceutical products, including vaccines and unnecessary medical treatments -- that are literally killing us.
The VAERS system uses a version of the "data exhaust" method, a data science approach by which large quantities of rough and even unreliable data are sifted in search of pattern. The individual data points, as the VAERS builders clearly state, have not been verified, are self-reported cases, and are not the point of the VAERS approach. It is an early warning system, not a precise instrument of measure.
Even if the data were confirmed, "Learn The Risk" may have selected VAERS case files that skew young and unhealthy, instead of taking a representative sample of all reports. A spot-check of 10 random cases of the 28 listed revealed that many of those listed had pre-existing serious health conditions and were residents of long term care facilities.
Lead Stories conducted a spot check of 10 of the 28 the cases in the list posted to Facebook to verify they are authentic items in the VAERS database. They are.
The report ID numbers correspond to actual VAERS reports and the details of these reports; age, sex, date of vaccination, date of death, and the vaccine that had been administered were, in the spot check, all accurate. But, digging into the VAERS database, it was clear "Learn the Risk" had selected young patients with serious health problems. In many cases it was clear the decedent, despite their relatively young age, was a resident of a long-term care facility and most reports listed multiple pre-existing health problems. The patient's medical history, and list of medications some were taking at the time, were included in most of the VAERS reports, but absent from the list shared in the Facebook post.
the "Learn the Risk" post on Facebook leaves out important context: The primary purpose of the VAERS reporting system is for 'rapid signal detection for rare adverse events'. In other words, as a crowd-sourced data set, it is only useful in the aggregate and less so in the details of each un-corroborated report.
The instructions included in the post for how to search the database may be difficult to follow. The CDC has a 13-minute video (here) which explains how to navigate the search functions. This video highlights some of the strengths and limitations of the VAERS data:
Strengths of VAERS data
- National data
- Accepts reports from anyone
- Collection information about vaccine, characteristics of person vaccinated, adverse event
- Data available to public
- Rapid signal detection for rare adverse events-
signals are potential safety issues that usually require more controlled studies to evaluate whether or not they represent an actual risk.Limitations of VAERS data
- Reporting bias
- Inconsistent data quality and completeness
- No unvaccinated comparison group
- Cannot calculate how often adverse events occur
- Generally cannot assess if vaccine caused an adverse event
The summary of the reporting system on the Wonder.CDC.gov website contains several additional considerations about the limitations of the VAERS data:
- Vaccine providers are encouraged to report any clinically significant health problem following vaccination to VAERS, whether or not they believe the vaccine was the cause.
- Reports may include incomplete, inaccurate, coincidental and unverified information.
- The number of reports alone cannot be interpreted or used to reach conclusions about the existence, severity, frequency, or rates of problems associated with vaccines.
- VAERS data are limited to vaccine adverse event reports received between 1990 and the most recent date for which data are available.
- VAERS data do not represent all known safety information for a vaccine and should be interpreted in the context of other scientific information.
Lead Stories reached out to the U.S. Food and Drug Administration by email to learn more about how VAERS reports that include death are investigated. Alison Hunt, the press officer at the FDA answered our question:
Notably, the reports to VAERS are unverified reports; the report of an adverse event to VAERS is not documentation that a vaccine caused the event. Additional details on the limitations of VAERS data can be found here: https://vaers.hhs.gov/data/dataguide.html.
If there were a rising number of deaths, it would attract government action, Hunt said:
FDA takes all reports of adverse events related to vaccines seriously, and in the case of COVID-19 vaccines, the agency, together with CDC, is actively engaged in safety surveillance of these vaccines... Any reports of death following the administration of vaccines are promptly and rigorously investigated jointly by FDA and CDC. Such an investigation includes working with health care providers to obtain medical histories and clinical follow-up information. Clinical case review is conducted by FDA and CDC. In addition, FDA and CDC have a wide array of systems in place to monitor the safety of vaccines. Details can be found here: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety.html.
As of the time of writing this Lead Stories report, no such spikes in the data have been detected, said the FDA spokesperson:
To date, we have not seen any new safety signals following administration of either authorized COVID-19 vaccine...There will be multiple, complementary systems in place with validated analytic methods that can rapidly detect signals for possible vaccine safety problems. The U.S. government has a well-established post-authorization/post-approval vaccine safety monitoring infrastructure that will be scaled up to meet the needs of a large-scale COVID-19 vaccination program. The U.S. government - in partnership with health systems, academic centers, and private sector partners - will use multiple existing vaccine safety monitoring systems to monitor COVID-19 vaccines in the post-authorization/approval period. Some of these systems are VAERS, the Vaccine Safety Datalink (VSD), the Biologics Effectiveness and Safety (BEST) Initiative, and Medicare claims data. Should any new safety signals be identified by FDA and CDC through this safety surveillance, that information will be communicated to the public.
Lead Stories recently published an article addressing the question about the statistical inevitability that some people who are vaccinated will die of unrelated causes:
A certain number of people can be expected to die in any given week, for any given reason. Assuming that a particular death or illness is caused by the vaccine because it was administered just before someone got sick is an example of a logical fallacy. The fact that one event precedes the other does not necessarily mean that the first event caused the second. Correlation and causation are not the same thing.
Obviously, as people get older, their chance of dying increases. That's true regardless of the vaccine. The hope with the shot is that it will lower the number of people who contract the disease and thereby decrease the number of additional illnesses and deaths.