Did Albert Bourla, the CEO of biopharmaceutical company Pfizer, say that the company's COVID-19 treatment pill will contain a microchip that transmits information once swallowed? No, that's not true: A Pfizer spokesperson told Lead Stories that the claim is false. Additionally, a 2018 clip of Bourla discussing ingestible technology is unrelated to Paxlovid, Pfizer's COVID treatment pill.
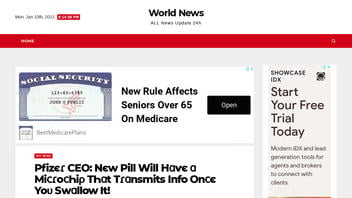
The claim appeared in an article (archived here) published on January 3, 2022, by breaking99times.com. The article was titled:
Pfizєɾ CEO: Nєw Pill Will Hɑѵє ɑ MiᴄɾoᴄҺiρ TҺɑt Tɾɑnsmits Info Onᴄє Yoυ Swɑllow It!
The article implied that Bourla discussed Pfizer's COVID treatment pill -- which was approved for emergency use authorization by the Food and Drug Administration (FDA) on December 22, 2021 -- in the context of ingestible technology. Ingestible technology, also known as a "smart pill," allows patients and health care providers to track health conditions. The article included a clip of Bourla discussing ingestible technology while on a panel in 2018.
This is how the post looked on January 10, 2022:
(Source: Screenshot taken on Mon Jan 10 20:19:57 2022 UTC)
In an email to Lead Stories on January 10, 2022, Keanna Ghazvini, senior associate for global media relations at Pfizer, told us that the claim is not true.
Also, the evidence in the article to support its claim is unrelated to Paxlovid. The clip of Bourla on the panel was taken during the 2018 World Economic Forum, which occurred well before the start of the COVID-19 pandemic. The panel, called "Transforming Health in the Fourth Industrial Revolution," can be found here. Beginning at the 45:25 mark, an audience member asked the panel members for their thoughts on technology that helps engage patients. Bourla, who answered first, described Abilify MyCite, a smart pill approved by the FDA in 2017 that can track when the patient took the drug. Although he did not mention Abilify MyCite by name, Bourla brought up the drug to acknowledge advances in the relationship between technology and patient engagement. Paxlovid was not discussed in the clip.
The emergency use authorization issued by the FDA for Paxlovid does not mention a microchip. A section of the press release announcing Paxlovid's authorization, linked again here, describes how the product works:
Paxlovid consists of nirmatrelvir, which inhibits a SARS-CoV-2 protein to stop the virus from replicating, and ritonavir, which slows down nirmatrelvir's breakdown to help it remain in the body for a longer period at higher concentrations. Paxlovid is administered as three tablets (two tablets of nirmatrelvir and one tablet of ritonavir) taken together orally twice daily for five days, for a total of 30 tablets. Paxlovid is not authorized for use for longer than five consecutive days.
Lead Stories has published fact checks related to Bourla before. They can be found here.