Will the proposed Dietary Supplement Listing Act of 2022 ban all non-Food and Drug Administration (FDA)-approved supplements and ban all herbs? No that's not true: Senate Bill 4090 doesn't give the FDA more power. The bill would make ingredient lists available and require any supplements sold across state lines to be registered with the FDA.
The claim appeared in an Instagram post with a video on October 27, 2022. The in-video text referenced "Bill S 4090" and the caption with the video read:
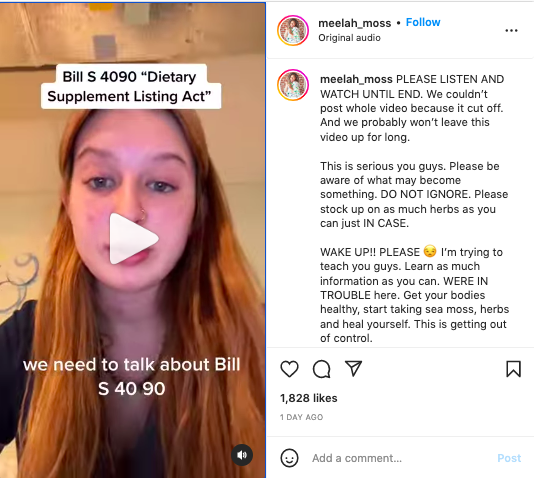
PLEASE LISTEN AND WATCH UNTIL END. We couldn't post whole video because it cut off. And we probably won't leave this video up for long.
This is serious you guys. Please be aware of what may become something. DO NOT IGNORE. Please stock up on as much herbs as you can just IN CASE.
WAKE UP!! PLEASE 😒 I'm trying to teach you guys. Learn as much information as you can. WERE IN TROUBLE here. Get your bodies healthy, start taking sea moss, herbs and heal yourself. This is getting out of control.
This is what it looked like at the time of writing:
(Image source: Instagram screenshot taken on Fri Oct 28 19:54:45 2022 UTC)
The bill is about transparency, according to a press release by co-sponsor Dick Durbin, and making more information available to consumers about dietary supplements and herbs. The summary of the measure says:
... the responsible person must register with the FDA specified information about each dietary supplement to be offered for sale, including (1) a list of all ingredients required by regulation to appear on the label; (2) the conditions of use; (3) any warnings and precautions; (4) certain claims characterizing the relationship between certain nutrients in the supplement and a disease or a health-related condition; (5) the responsible person's contact information; and (6) the locations where the supplement is manufactured, packaged, labeled, or held.
For a supplement that is offered for sale on the date that is 18 months after this bill's enactment (the effective date), the responsible person must submit the required information to the FDA no later than 60 days after the effective date. A supplement that is not offered for sale on the effective date may not be offered for sale until the responsible person has submitted the required information.
The FDA must establish a system that provides a unique identifier for each registered dietary supplement and a publicly accessible electronic database that allows a user to obtain certain information about a registered supplement.
Similar to regulations that govern products categorized as food, the bill would require anyone seeking to sell products categorized as dietary supplements to register various pieces of information with the FDA such as the ingredients, the specific health-related claims being made, any warnings and precaution labels and information about the manufacturer of the product.
FDA approval is not required of each product, as a condition of sale and, contrary to some online claims, the bill would not re-designate herbal supplements as drugs."