Does a Pfizer document show that the pharmaceutical company's COVID-19 vaccine causes "devastating harm to the musculoskeletal system"? No, that's not true: The Pfizer-BioNTech COVID-19 vaccine has been shown to be safe and effective in preventing COVID-19, with the most common side effects being mild and temporary, including injection site pain, fatigue or fever. There is no credible evidence or official documentation from Pfizer suggesting that their vaccine causes severe damage to the musculoskeletal system.
The claim appeared in a post on Twitter (archived here) published by Naomi Wolf on May 16, 2023. The tweet said:
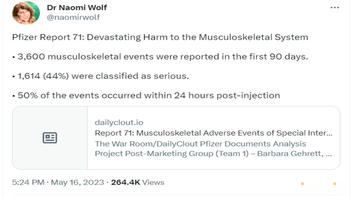
Pfizer Report 71: Devastating Harm to the Musculoskeletal System
• 3,600 musculoskeletal events were reported in the first 90 days.
• 1,614 (44%) were classified as serious.
• 50% of the events occurred within 24 hours post-injection
This is what the post looked like on Twitter at the time of writing: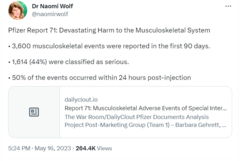
(Source: Twitter screenshot taken on Thu May 18 23:58:05 2023 UTC)
The tweet
The social media post links to an article on the DailyClout website called "Report 71." It's the DailyClout's analysis of a Pfizer document that was released as part of a Freedom of Information Act request by a group of more than 30 professors and scientists from universities including Harvard, Yale, Brown and UCLA. In their lawsuit filed September 16, 2021, the plaintiffs asked the U.S. Food and Drug Administration (FDA) to share the data it relied upon in licensing Pfizer's COVID vaccine.
The document, "5.3.6 CUMULATIVE ANALYSIS OF POST-AUTHORIZATION ADVERSE EVENT REPORTS OF PF-07302048 (BNT162B2) RECEIVED THROUGH 28-FEB-2021," was approved on April 30, 2021, according to the timestamp on the side of the online PDF. Lead Stories has also written multiple fact checks of claims about this particular Pfizer document.
The analysis
The information highlighted on Twitter and analyzed by DailyClout comes from a section of the Pfizer document (page 20) called "Musculoskeletal AESIs." The acronym stands for adverse events of special interest. A definition of the term is part of an FDA document on the "Optimisation of Safety Data Collection" for new drugs and medicines that are being licensed. This is how the agency defines AESIs:
These adverse events may warrant collection of additional information across the entire study population to better characterise these events (e.g., particular laboratory parameters; vital signs; risk factors; concomitant therapies; and/or concomitant illnesses).
At the time the Pfizer document was submitted, their vaccine was under emergency use authorization.
Vaccine Adverse Event Reporting System
Most of the numbers reported in the Pfizer document come from expert groups and regulatory authorities, including European health organizations and the Vaccine Adverse Event Reporting System (VAERS) in the United States, which is run by the Centers for Disease Control and Prevention and the FDA.
Lead Stories has debunked several claims about deaths and injuries that misuse VAERS data.
Anyone with internet access can add a report to the VAERS list of reports. The public access link to it expressly warns against unwarranted conclusions based on VAERS material because the list only provides a tally of unverified notes about any health event people experience after they are vaccinated.
The list itself cannot be used to prove or quantify, since all it shows is a chronological correlation, not the causal link that would be more difficult to establish. It's the equivalent of a police precinct's running "blotter" of reports that may serve as a starting point for police work, not an endpoint.
In addition, the Musculoskeletal section of the Pfizer document came to this conclusion on page 21:
This cumulative case review does not raise new safety issues. Surveillance will continue.
Pfizer response
In a May 19, 2023, email to Lead Stories, Pfizer media relations provided a response from the company regarding the claims in the social media post. It said:
We take adverse events that are potentially associated with our COVID-19 vaccine, BNT162b2, very seriously. We closely monitor all such events and collect relevant information to share with global regulatory authorities. It is important to note that serious adverse events that are unrelated to the vaccine are unfortunately likely to occur at a similar rate as they would in the general population. With hundreds of millions of doses of the Pfizer-BioNTech COVID-19 vaccine administered globally, the benefit-risk profile of our vaccine remains positive for all approved indications and age groups. Pfizer continues to monitor trial participants' health for two years after vaccination. In addition, we continue to perform post-authorization safety surveillance. Government and regulatory authorities, such as the Centers for Disease and Control (CDC), also conduct safety surveillance.
Vaccine 'well-established'
In a May 18, 2023, phone interview with Lead Stories, Dr. William Schaffner, a professor of infectious diseases at Vanderbilt University Medical Center, said COVID vaccines are "well-established" at this point. He added:
We now have given mRNA vaccines ... to millions upon millions of people. These vaccines are no longer new and unusual. They have been administered and their consequences have been studied now as thoroughly as any vaccine we have ever given ... The rare adverse events associated with these vaccines are very well known ... [and] that they [the vaccines] are remarkably effective in preventing severe disease and remarkably safe.
The CDC website says more than 672 million doses of COVID-19 vaccines were administered in the United States from December 14, 2020, through March 1, 2023. Vaccination is recommended for everyone 6 months and older.
About Naomi Wolf
Wolf is an author and activist. She is also the CEO and co-founder of the DailyClout, to which her social-media post links. Her Twitter and YouTube accounts were suspended in 2021 for sharing vaccine misinformation.Wolf later was reinstated to Twitter. Her description includes this line: "Deplatformed 7 times, still right."
She has a doctorate in English language and literature/letters, according to her LinkedIn profile, but holds no medical degree.
Other Lead Stories fact checks involving Naomi Wolf can be found here.