Does the German government have study figures that show people given the Pfizer COVID-19 vaccine (Comirnaty) "have overall suffered from fewer medically undesirable events as subjects who received the placebo"? No, that's not true: As of August 4, 2023, Germany's Federal Ministry of Health said it did not have "such figures." But not having such study figures proves nothing in regards to the vaccine -- one medical expert told Lead Stories the German government was responding to a "provocative question."
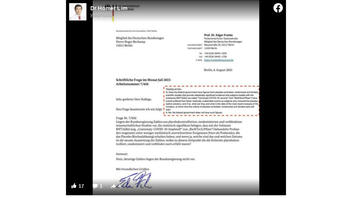
The claim appeared in a post (archived here) published on Facebook by "Dr Homer Lim" on August 9, 2023. Lim is a Philippines-based practitioner of alternative medicine and is trained in complementary and integrative medicine, according to his website. The post features a letter from the German Federal Ministry of Health to a member of the German parliament, answering a question about studies involving the Pfizer COVID vaccine. This is the question and answer as officially translated in the letter:
Q: Does the federal government have figures from placebo-controlled, randomized and blinded scientific studies that provide statistically significant evidence that subjects treated with the substance BNT1622 (so-called 'Comirnaty-COVID-19 vaccine* from "BioNTech/Pfizer*) have overall suffered from fewer medically undesirable events as subjects who received the placebo (saline solution), and if so, what are they and what is the date of the most recent analysis of the numbers, at which time the criteria of placebo-controlled, randomized and blinded were still met?
A: No, the federal government does not have such figures.
This is what the post looked like on Facebook at the time of writing:
(Source: Facebook screenshot taken on Wed Aug 23 21:23:31 2023 UTC)
German Federal Ministry of Health
Taken on its own, the Q&A in the letter from the German Federal Ministry of Health seems to suggest that the Pfizer COVID vaccine hasn't met regulatory muster, but that's not the case. In an August 15, 2023, email to Lead Stories, ministry press officer Parissa Hajebi said:
Comirnaty® has been authorised centrally by the European Commission for the European Union.
Details on the vaccine's safety and efficacy can be found on the website of the European Medicines Agency.
Paul-Ehrlich-Institut
Susanne Stöcker, a press officer at the Paul-Ehrlich-Institut (PEI), Germany's federal institute for vaccines and biomedicines, said in an August 18, 2023, email to Lead Stories that the question posed in the letter is "not a valid one." She continued:
It is not biologically possible to have fewer adverse events in the drug group than in the placebo group.
An adverse event can be a true adverse reaction, also known as a side effect related to the vaccine, or a coincidental event following vaccination.
Additionally, while the letter in the social media post said the German federal government does not have the figures asked about in the letter, the PEI does. Stöcker added:
The regulatory authorities, including the Paul-Ehrlich-Institut, which is part of the Federal Ministry of Health, have study data on the vaccine Corminaty and placebo (saline) that meet the criteria of placebo-controlled, randomised and blinded.
Pfizer
In an August 11, 2023, email response to Lead Stories from Pfizer media relations, the vaccine maker said it couldn't "speak to the government document specifically," but added:
[O]ur data pre and post licensure have been, and are, extensively reviewed by multiple regulatory and advisory bodies around the world. The Pfizer-BioNTech COVID-19 vaccine has been administered to hundreds of millions of adults, adolescents and children generating robust data demonstrating a favorable safety profile and high level of protection against severe COVID-19 disease and hospitalization.
Vaccine expert
To get some perspective, Lead Stories contacted Dr. William Schaffner, a professor of medicine in the division of infectious diseases at Vanderbilt University Medical Center. He was also a member of the COVID vaccines work group, the subcommittee that dealt with COVID vaccines on behalf of the U.S. Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices. In an August 23, 2023, phone interview, this was Schaffner's response to the ministry's letter in the social media post:
Obviously, the Minister of Health was sent a provocative question by a member of the Bundestag, the German legislature, and I thought the response was entirely unhelpful, unfortunately. What I would have said is, first of all, there were, prior to licensure, prospective-controlled, placebo-controlled trials. That was for the initial vaccine.
Since the release of the vaccine, countries have used surveillance systems to track adverse events. In the United States, it's called the Vaccine Adverse Event Reporting System, or VAERS.
Vaccine Adverse Event Reporting System
VAERS, which the CDC co-sponsors with the U.S. Food and Drug Administration, operates as a crude early warning system and not as a database for the quantification of specific outcomes following vaccination.
Anyone with internet access can add a report to the VAERS list of reports. The public access link to it expressly warns against unwarranted conclusions based on VAERS material because the list only provides a tally of unverified notes about any health event people experience after they are vaccinated.
The list itself cannot be used to prove or quantify, since all it shows is a chronological correlation, not the causal link that would be more difficult to establish. It's the equivalent of a police precinct's running "blotter" reports that may serve as a starting point for police work, not an endpoint.
More Schaffner
Schaffner said the surveillance systems have been "very effective" in finding "very rare adverse events" that included myocarditis in young adults and older adolescents with mRNA vaccines and blood clots in women with the Johnson & Johnson/Janssen vaccine. He called them "needles in a haystack that were quickly defined, widely publicized, both to the profession and to the general public." In concluding his response to the letter from the German ministry of health, Schaffner said:
So, the ongoing safety surveillance system that exists country by country throughout Europe in Canada, in the United States, all contribute ongoing, reassuring information regarding the safety of these vaccines I think that would have been a much more complete answer.
Additional Lead Stories fact checks of claims about COVID-19 vaccines can be found here.