Did the Food and Drug Administration say all cancer treatments "actually cause cancer"? No, that's not true: The agency issued a press release announcing that it is investigating only cases related to one specific type of drugs -- CAR-T therapies. Medical professionals who discussed the press release with credible media organizations said the chances of developing a second cancer as a result of such therapy are low and that the benefits outweigh the potential risks, given that CAR-T treatments are only prescribed after other lines of therapy fail.
The claim appeared in a post (archived here) on Instagram on December 7, 2023. It opened:
😢 Killing us off slowly.
The shared image continued:
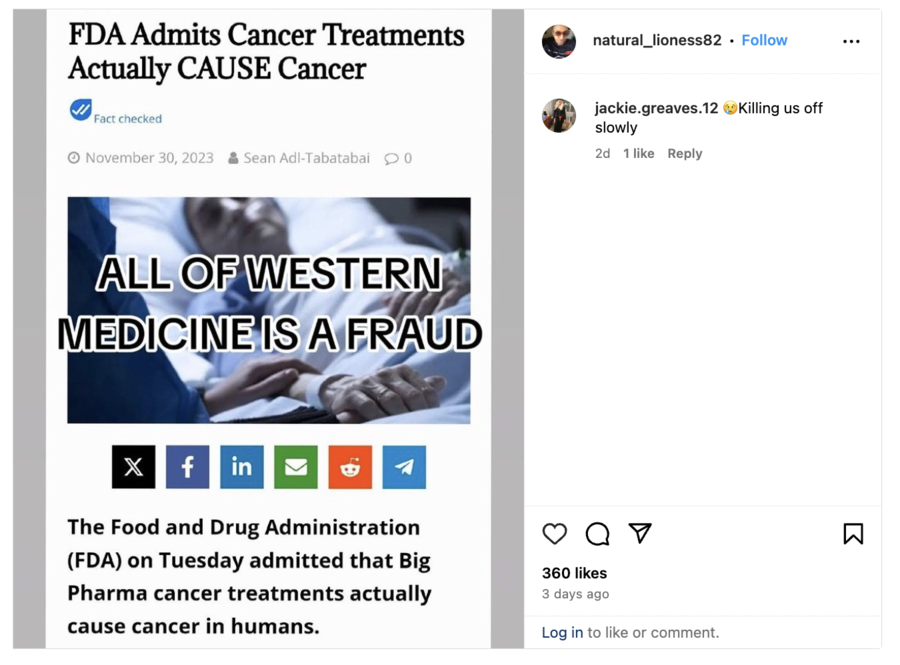
FDA Admits Cancer Treatments Actually CAUSE Cancer. ALL OF WESTERN MEDICINE IS A FRAUD.
This is what the post looked like on Instagram at the time of writing:
(Source: Instagram screenshot taken on Mon Dec 11 15:18:21 2023 UTC)
The post contained a screenshot of an article (archived here) published on November 30, 2023, by The People's Voice, a source of false claims debunked by Lead Stories numerous times over the years.
The article referred to a November 28, 2023, press release (archived here) from the Food and Drug Administration (FDA).
An FDA spokesperson confirmed to Lead Stories via email on December 11, 2023, that the agency's press release discussed CAR-T therapies, not all cancer treatments:
Since approval of the first CAR-T therapies in 2017, as of Nov. 29, 2023, the FDA has received 20 reports (15 FAERS reports and 5 cases from clinical trials) of T cell malignancy, which suggest that T cell malignancy is an identified risk for approved BCMA-directed and CD19-directed genetically modified autologous CAR T cell immunotherapies.
Medline, a service of the National Library of Medicine, describes CAR-T therapies (archived here) as one type of approach that "combines the technologies of gene therapy and cell therapy" so that "modified immune cells can specifically attack cancer cells."
The National Cancer Institute (archived here) wrote:
... immune system-boosting drugs have shown the ability to shrink, and even eradicate, tumors in some people with advanced cancer. In a small percentage of patients, these treatment responses can last for years.
CAR-T therapies are typically used in cases of advanced blood cancers such as lymphomas, lymphoblastic leukemia or myeloma when other treatments hadn't worked.
The FDA press release said this class of drugs was already known for higher risks of causing second cancers and carried a warning about it.
Eric Smith, an oncology researcher at the Dana-Farber Cancer Institute and assistant professor at Harvard Medical School, told (archived here) Harvard University's website that it is too early to draw any conclusions about what data there is:
The theoretical risk is that the virus will integrate right in front of a gene associated with cancer, and the promoter -- that gets the CAR to be made from the DNA inside the T cell -- can increase production of the oncogene. ...
It's unclear if that theoretical risk is actually what occurred. These are blood cancer patients who have received other therapies, are immunosuppressed, and are already predisposed to developing second blood cancers.
Smith estimated (archived here) that the overall number of patients treated with CAR-T drugs has reached roughly 35,000, meaning that the reports the FDA is investigating account for 0.06 percent of all patients.
This estimate is comparable with the frequency of second cancers as a result of chemotherapy and radiation: 1 percent or less, as Lead Stories previously wrote.
Medical doctors interviewed by the Lancet (archived here) also estimated the odds of second cancers resulting from CAR-T therapies as low and added that those who get prescribed this treatment didn't have any options at all before it existed.
Other Lead Stories fact checks about health can be found here.