Does material plucked from the European Union's list of reports of possible drug reactions justify an essay's claim that vaccine side effects have killed two people for every three who were saved from dying of COVID-19? No, that's not true: The essay did not pass through customary scientific peer review and editing. Staff members of the journal it appeared in have posted a letter saying the paper misrepresents vaccination efforts and vaccine safety data and the journal was, as of June 28, 2021, deciding what to do about the problems with the article, which caused a member of its editorial board to resign. The article is based in part on the unverified data in the European Union Adverse Drug Reactions (EURDA) data base. Users are clearly told the reports in it have not been verified and cannot be used to quantify causes of death or injury.
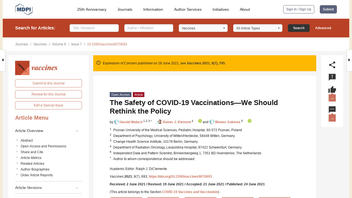
The claims made by social media users about COVID vaccine-caused deaths refer to an article published June 24, 2021, by open access publisher Vaccines titled "The Safety of COVID-19 Vaccinations--We Should Rethink the Policy" (archived here) which opened:
For three deaths prevented by vaccination, we have to accept one inflicted by vaccination.
Users on social media only saw this title, description and thumbnail:
The Safety of COVID-19 Vaccinations--We Should Rethink the Policy
Background: COVID-19 vaccines have had expedited reviews without sufficient safety data. We wanted to compare risks and benefits. Method: We calculated the number needed to vaccinate (NNTV) from a large Israeli field study to prevent one death. We accessed the Adverse Drug Reactions (ADR) database of the European Medicines Agency and of the Dutch National Register (lareb.nl) to extract the number of cases reporting severe side effects and the number of cases with fatal side effects. Result: The NNTV is between 200-700 to prevent one case of COVID-19 for the mRNA vaccine marketed by Pfizer, while the NNTV to prevent one death is between 9000 and 50,000 (95% confidence interval), with 16,000 as a point estimate. The number of cases experiencing adverse reactions has been reported to be 700 per 100,000 vaccinations. Currently, we see 16 serious side effects per 100,000 vaccinations, and the number of fatal side effects is at 4.11/100,000 vaccinations. For three deaths prevented by vaccination, we have to accept one inflicted by vaccination. Conclusions: This lack of clear benefit should cause governments to rethink their vaccination policy.
A Michigan doctor and professor of medicine reposted a link to the study and called the findings "horrific" after characterizing them as "Science. Statistics. Analysis. Largest database in the world. OMG. For every 3 Covid deaths prevented by vaccination, 2 vaccine induced deaths occur."
But the editors of Vaccines published on June 28, 2021, an "Expression of Concern" to alert readers to the problems also identified by Lead Stories staff:
The journal is issuing this expression of concern to alert readers to significant concerns regarding the paper cited above [1].
Serious concerns have been raised about misinterpretation of the data and the conclusions.
The major concern is the misrepresentation of the COVID-19 vaccination efforts and
misrepresentation of the data, e.g., Abstract: "For three deaths prevented by vaccination
we have to accept two > inflicted by vaccination". Stating that these deaths linked to vaccination efforts is incorrect and distorted.
The point of EURDA, like the U.S. version called VAERS, is to quickly accumulate masses of informal reports during a vaccination drive, which vaccine safety teams scan for patterns. If a pattern emerges, researchers then must confirm the authenticity of the reports. For the true and correct reports, investigators must obtain death certificates, medical records and other detailed information to determine if the deaths were vaccine-caused or coincidental. Lead Stories has contacted the European Medicines Agency for comment on the authors' use of the rough reports collected by its Adverse Drug Reactions (ADR) database and will add its comments, if appropriate, when the agency replies.
On the ADR website, the agency provides a guide to interpretation of spontaneous case reports of suspected adverse reactions to medicines that clearly identifies the problems with the analysis in the Vaccines article:
- Case reports of suspected adverse reactions alone are rarely sufficient to confirm that a certaineffect in a patient has been caused by a specific medicine.
- The fact that a suspected adverse reaction has been reported does not necessarily mean that themedicine has caused the observed effect as this could have also been caused by the disease beingtreated, a new disease the patient developed, or by another medicine that the patient is taking.
- Case reports need therefore to be assessed by an expert.
- A single case report should be seen as a piece of a jigsaw puzzle, where further data are usuallyneeded to complete the picture. These include data from worldwide spontaneous case reports,clinical trials and epidemiological studies.
An associate editor of Vaccines quit the magazine over the article. Prof. Florian Krammer, a biotechnology Ph.D. who runs a lab that studies viruses and vaccines at the Icahn School of Medicine in New York City, posted his resignation on Twitter.
Hi John, I was made aware of the paper yesterday, read it, sent an email to the editorial staff with exactly your question and then resigned from my role at the journal.
-- Florian Krammer (@florian_krammer) June 26, 2021