Did the CDC's PCR test fail to distinguish flu from COVID and did it cause errors in Centers for Disease Control and Prevention tallies by which the agency tracked the spread of SARS-CoV-2 infections? No, that's not true. The claim was made without providing evidence. The CDC says the "Real-Time PCR test" was the first available diagnostic tool, worked well and was replaced by faster and more sensitive tests, but was not error-prone. Independent of the CDC, a UCLA lab expert said there were no issues with accuracy of the test. The CDC published an alert July 21, 2021, announcing that any labs still using the test that the agency had released in February 2020 should shift to one of the newer, faster tests.
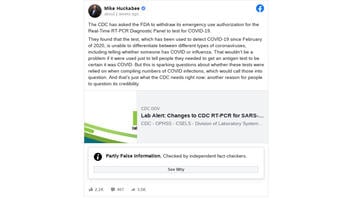
The claim originated in a July 26, 2021, Facebook post (archived here) published by former Arkansas Gov. Mike Huckabee under the title "The CDC has asked the FDA to withdraw its emergency use authorization for the Real-Time RT-PCR Diagnostic Panel to test for COVID-19.". It opened:
They found that the test, which has been used to detect COVID-19 since February of 2020, is unable to differentiate between different types of coronaviruses, including telling whether someone has COVID or influenza ... this is sparking questions about whether these tests were relied on when compiling numbers of COVID infections, which would call those into question.
This is what the post looked like on Facebook at the time of writing:
(Source: Facebook screenshot taken on Tue Aug 10 15:58:34 2021 UTC)
Although the CDC 2019 Novel Coronavirus (2019 nCoV) Real-Time RT-PCR Diagnostic Panel met an important unmet need when it was developed and deployed and has not demonstrated any performance issues, the demand for this test has declined with the emergence of other higher-throughput and multiplexed assays.The CDC Novel Coronavirus (2019 nCoV) Real-Time RT-PCR is a highly accurate test; the decision to discontinue support for the EUA was not based on the test's performance.
Fulce said that as the pandemic continued, new tests were developed that are faster, which is why the CDC is no longer pursuing Emergency Use Authorization, the bureaucratic process by which the FDA brings new drugs and medical tests online.
CDC's RT-PCR to detect SARS-CoV-2, the virus that causes COVID-19, was one of the first tests available. This test met an immediate need when it was developed and was initially in high demand. Commercial manufacturers have developed other tests since this test was first introduced. These newer tests are faster, have higher throughput and are designed to be more efficient. This has decreased demand for CDC's test has declined significantly.
She said the CDC is encouraging use of commercial products. "There are many readily available commercial molecular diagnostic tests that detect the genetic material of the virus that causes COVID-19. A list of alternative molecular diagnostic tests are available at In Vitro Diagnostics EUAs - Molecular Diagnostic Tests for SARS-CoV-2 | FDA.CDC is encouraging public health labs to adopt the CDC Influenza SARS-CoV-2 (Flu SC2) Multiplex assay for surveillance testing for both SARS-CoV-2 (the virus that causes COVID-19) and influenza. This is a very accurate test that will save both time and resources for the lab. The SARS-CoV-2 (Flu SC2) Multiplex Assay tests for SARS-CoV-2 and for influenza, independently. The test will specify whether a sample tested positive for SARS-CoV-2, influenza or both."
Huckabee is wrong about the test the CDC released for use early in the epidemic, said Prof. Omai Garner, director of Point of Care Testing at the UCLA Department of Pathology and Laboratory Medicine. A Ph.D. in biomedical sciences and board-certified by the American Board of Medical Microbiology, Garner wrote in an August 5, 2021, email to Lead Stories that UCLA Health put the RT-PCR test through its paces during the early waves of the pandemic and it "We were running that test at UCLA Health at the start of the pandemic. The original CDC PCR test can easily distinguish between seasonal flu and COVID."
The CDC announced on July 21, 2021, that it would not pursue full approval by the FDA for the test.