Is the Pfizer COVID-19 vaccine not approved by the FDA? No, that's not true: The Food and Drug Administration approved the Pfizer-BioNTech COVID-19 vaccine on August 23, 2021. The agency made the announcement publicly, sharing the approval letter to the pharmaceutical company as well as posting the approval in the "Fact Sheet for Health Care Providers Administering Vaccine (Vaccination Providers)" on the agency's website.
The false claim was made on an internet show, which later issued a retraction, admitting they were "wrong" and the Pfizer-BioNTech COVID-19 vaccine is FDA-approved.
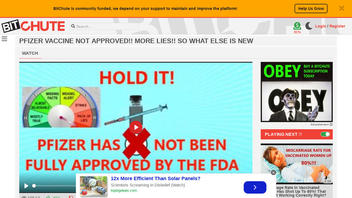
The claim appeared in a Bitchute video published on August 24, 2021, titled: "PFIZER VACCINE NOT APPROVED!! MORE LIES!! SO WHAT ELSE IS NEW" (archived here), which opened:
It is still under emergency use. Source: RedPil78 Whats really happening? Find out with these videos: A combat veteran gives his city leaders a warning about what is to come. https://www.bitchute.com/video/Skwe2x8SQmjL/ $500 TRILLION Lawsuit ...
Users on social media only saw this title, description and thumbnail:
PFIZER VACCINE NOT APPROVED!! MORE LIES!! SO WHAT ELSE IS NEW
It is still under emergency use. Source: RedPil78 Whats really happening? Find out with these videos: A combat veteran gives his city leaders a warning about what is to come. https://www.bitchute.com/video/Skwe2x8SQmjL/ $500 TRILLION Lawsuit ...
The Pfizer-BioNTech COVID-19 was fully approved by the FDA for people 16 years of age and up on August 23, 2021.
The video on Bitchute is from the website Red Pill News and their internet show "Occam's Razor." Host Craig Mason made the false claim on August 23, 2021, hours after the FDA approved the Pfizer vaccine:
So, what I have here is the actual letter from the Food and Drug Administration. The FDA. Delivered to Pfizer and dated on today. Now, it summarizes the reasoning for enacting the emergency use authorization act in the first place. It goes through all of the steps that they've taken, the studies that they've done. It says you can only use it for people 16 years of age and over which kind of makes you wonder why they're trying to give it to children under the age of 16. But if you scroll down never once does it say that they are changing the emergency use authorization, it just says they are continuing it. So this has not been approved by the FDA.
This is not true.
Page 2 of the letter the FDA sent to Pfizer clearly states:
On August 23, 2021, FDA approved the biologics license application (BLA) submitted by BioNTech Manufacturing GmbH for COMIRNATY (COVID-19 Vaccine, mRNA) for active immunization to prevent COVID-19 caused by SARS-CoV-2 in individuals 16 years of age and older.
On August 24, 2021, the show aired a correction, admitting they were wrong and that the vaccine did receive full approval from the FDA for people 16 and up. The update was aired and posted on Rumble.com.
The video had a caption on screen that read, "I screwed up OCCAM'S RAZOR Episode 117 with Zak Paine and Craig Mason." It opened:
Good afternoon, patriots. And welcome to another episode of 'Occam's Razor.' As you can probably see from the title, I have a major retraction to make about my video from late last night. I got excited, I'm not a scientist, I did not understand the language, I interpreted it incorrectly and I need you guys to know that. I changed the title on the video, I added a disclaimer and I just want to make sure I am absolutely clear. It's super important to keep yourself accountable. But it's not unheard of for people to get things wrong.
The caption below the video on Rumble also reiterated the claim was not true:
In my second episode of 'Red Pill News' last night, I incorrectly stated that the Pfizer vaccine had not actually been approved. I was wrong. The EUA has been extended for some age groups, while the approval was given for others, despite the fact that studies have not been completed and they even list dates for when they must be to achieve approval. It's unprecedented and the letter was intentionally vague. Also, I'm not a scientist, but that's not a good enough excuse. I'm admitting fault and taking responsibility."
The "FACT SHEET FOR HEALTHCARE PROVIDERS ADMINISTERING VACCINE (VACCINATION PROVIDERS)" posted on the FDA website clearly states that the vaccine is approved on Page 1:
COMIRNATY (COVID-19 Vaccine, mRNA) is an FDA-approved COVID-19 vaccine made by Pfizer for BioNTech. It is approved as a 2-dose series for the prevention of COVID-19 in individuals 16 years of age and older and is also authorized for emergency use in individuals 12 through 15 years and to provide a third dose to individuals 12 years of age and older who have been determined to have certain kinds of immunocompromise.