STORY UPDATED: check for updates below.
Did the FDA (Food & Drug Administration) redact a COVID-19 vaccine ingredient because it's a mysterious substance that the public shouldn't know about? No, that's not true: The redacted ingredient is water. A spokesperson for the FDA told Lead Stories that if the company considers the ingredient a trade secret or a confidential commercial item, the ingredient gets redacted on documents that will be seen by the public. In this case, neither the maker nor the FDA has, as of the time this was updated, explained why water got listed as a redacted ingredient.
The claim appeared in an Instagram post (archived here) where it was published on the page of vaccine-skeptical Physicians for Informed Consent on September 21, 2021. The first sentence of the caption read:
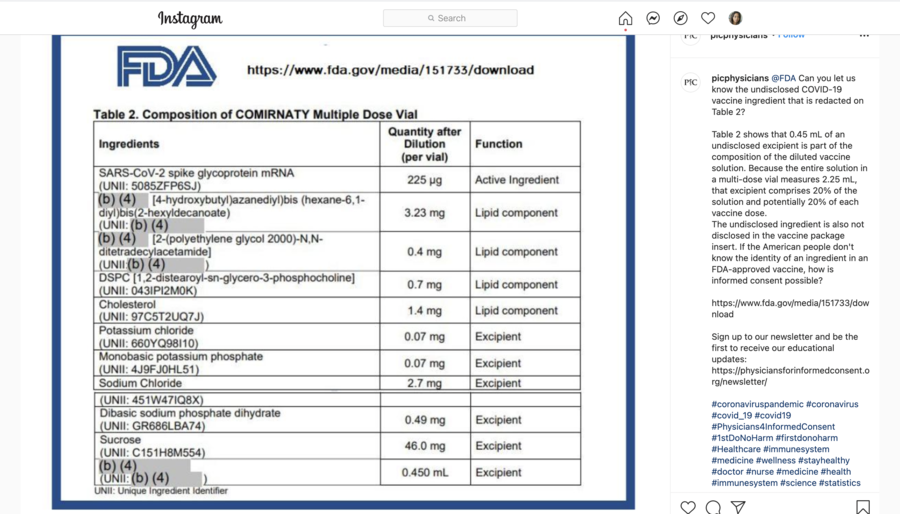
@FDA Can you let us know the undisclosed COVID-19 vaccine ingredient that is redacted on Table 2?
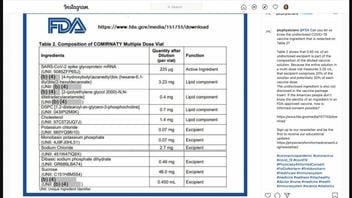
This is what the Instagram post looked like at the time of Lead Stories' original fact check:
(Source: Instagram screenshot taken on Wed Sep 22 15:08:34 2021 UTC)
The FDA spokesperson told Lead Stories in a September 22, 2021 email that any person interested in seeing an unredacted ingredient list of the COVID-19 vaccine from the FDA can file a Freedom of Information Act, or FOIA, request.
Lead Stories did the research to find the redacted ingredient. Using a publicly available European Medicines Agency document titled, "Comirnaty, INN COVID-19 mRNA Vaccine (nucleoside-modified)," the ingredients of the COVID-19 vaccine were found on the 13th page of the 40-page document. Comirnaty is the trade name for the Pfizer BioNTech vaccine, according to the FDA. We found that the only ingredient that was mentioned in the document, but not in the Instagram post was "Water for injections".
(Source: Comirnaty, INN COVID-19 mRNA Vaccine (nucleoside-modified) screenshot taken on Thu Sep 23 15:09:55 2021 UTC)
Lead Stories contacted Pfizer, maker of the vaccine, asking why water was redacted from the ingredient list. A Pfizer spokesperson replied September 22, 2021 sending us a document with the ingredients on page 3. When we followed up, later that same day, by asking why water was not present on the ingredient list, we received no answer. We will update this story if we receive a response from Pfizer.
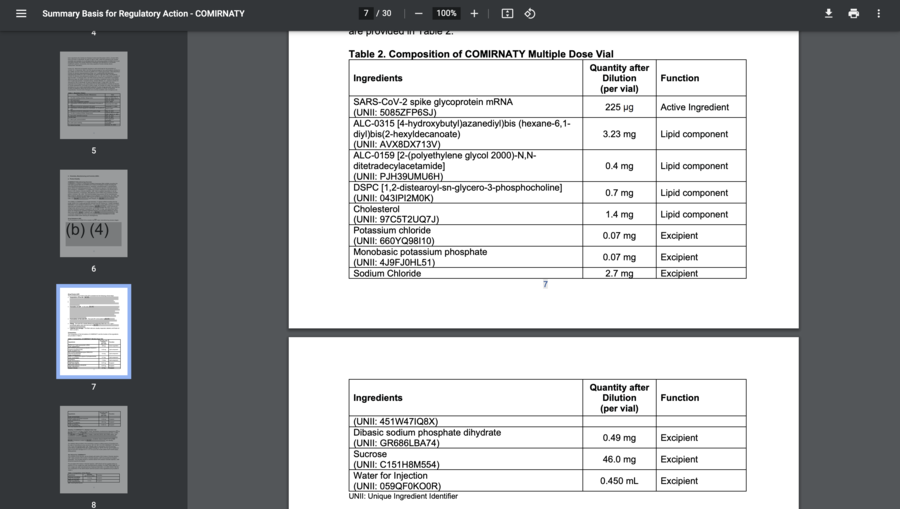
An FDA press officer sent Lead Stories the same document that can be seen in the Instagram post on September 30, 2021. An unredacted ingredient list can be found on page 7 and 8 of the document. The FDA press officer told us, "Following up to let you know FDA has determined that additional information could be released in the Comirnaty Summary Basis for Regulatory Action (SBRA), and has posted the updated document on its web site: Summary Basis of Regulatory Action (pages 7 and 8)". The last redacted ingredient on the list is labeled as "Water for injections" with a 0.450 mL quantity in the Cominarty multiple dose vial.
(Source: FDA.gov screenshot taken on Thur Sep 30 9:19:34 2021 UTC)
After Lead Stories published its initial fact check, which mistakenly identified the water as salt water, Physicians for Informed Consent corrected its original post, while still asking the FDA to explain why that ingredient had been at one time redacted.
Updates:
-
2021-10-05T22:22:48Z 2021-10-05T22:22:48Z CORRECTION: Drawing an inference from Comirnaty's package insert, Lead Stories' original fact check misidentified the redacted ingredient as sodium chloride, or salt water. Subsequent documents provided by the vaccine maker and FDA clarified the redacted ingredient was plain water.