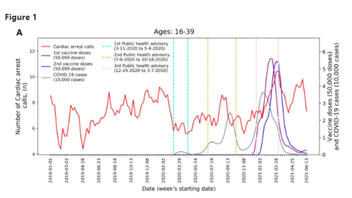
Did Israel's under-40 population experience an increase in "emergency cardiovascular events" during the rollout of COVID-19 vaccines and the third wave of the virus? No, there's no evidence to support that. While data show an increase in calls to emergency medical services (EMS) for these types of events, it's unclear whether the final diagnoses of these cases represent "cardiac arrest and/or acute coronary syndromes at all," according to Dr. Jason H. Wasfy, a cardiologist at Massachusetts General Hospital in Boston.
The claim appeared in an article (archived here) published by Scientific Reports on April 28, 2022, titled "Increased emergency cardiovascular events among under-40 population in Israel during vaccine rollout and third COVID-19 wave - Scientific Reports." It opened:
Cardiovascular adverse conditions are caused by coronavirus disease 2019 (COVID-19) infections and reported as side-effects of the COVID-19 vaccines. Enriching current vaccine safety surveillance systems with additional data sources may improve the understanding of COVID-19 vaccine safety. Using a unique dataset from Israel National Emergency Medical Services (EMS) from 2019 to 2021, the study aims to evaluate the association between the volume of cardiac arrest and acute coronary syndrome EMS calls in the 16-39-year-old population with potential factors including COVID-19 infection and vaccination rates. An increase of over 25% was detected in both call types during January-May 2021, compared with the years 2019-2020. Using Negative Binomial regression models, the weekly emergency call counts were significantly associated with the rates of 1st and 2nd vaccine doses administered to this age group but were not with COVID-19 infection rates. While not establishing causal relationships, the findings raise concerns regarding vaccine-induced undetected severe cardiovascular side-effects and underscore the already established causal relationship between vaccines and myocarditis, a frequent cause of unexpected cardiac arrest in young individuals. Surveillance of potential vaccine side-effects and COVID-19 outcomes should incorporate EMS and other health data to identify public health trends (e.g., increased in EMS calls), and promptly investigate potential underlying causes.
This is what the post looked like on the Scientific Reports website on May 4, 2022:
(Source: Scientific Reports screenshot taken on Wed May 4 15:08:11 2022 UTC)
The limitations
In a May 5, 2022, email to Lead Stories, Wasfy, who is also a member of the American College of Cardiology Science and Quality Committee, said the study has "multiple important analytic limitations and lacks important clinical context":
First, the primary outcomes are calls to emergency medical services (EMS) for cardiac arrest and acute coronary syndrome. However, those diagnoses are often not made until labs and radiology are available from the hospitalization afterward. As such, it is unclear that the main outcomes in this study really represent cardiac arrest and/or acute coronary syndromes at all.
Furthermore, the authors compared large groups of people over time without knowing which individuals who had covid or covid vaccination. If more young people in Israel as a whole really did have cardiac arrest and/or acute coronary syndromes in 2021 relative to 2020 or 2019, there were many other potential causes for those changes over time aside from covid vaccination. Attributing these changes to covid vaccination is really speculative - it could be many other things.
In a May 4, 2022, email to Lead Stories, Dr. Matt Robinson, an infectious disease specialist at Johns Hopkins Medicine, suggested the focus of the study was cherry-picked:
The authors of this manuscript have attempted to correlate the number of calls made to emergency services in Israel for cardiac arrests and heart attacks in persons age 16-39 with COVID-19 vaccination campaigns. Calls for ambulances for cardiac arrests and heart attacks are luckily uncommon in the young, but as expected, the numbers of such calls vary week to week. By cherry picking time intervals, the authors find a very weak correlation between COVID-19 vaccination and such calls. They then highlight an increase in these calls in the first few months of 2021 when COVID-19 vaccination was ongoing compared to 2020, before they were available. However, their own data also shows that calls increased in the beginning of 2020 compared to 2019 - both years in which COVID-19 vaccination was not available. Because the analysis only looks at total tallies of calls and vaccine doses administered, there is no way to know that the people who had cardiac arrests and heart attacks were the ones that actually received vaccines. It is easy to find false correlations in data trends if you look for them.
The language
Continuing in his email, Wasfy called the language of the study "often confusing and alarmist":
For example, one of the main results is 'an increase of over 25%' in cardiac arrest among young people in 2021. These numbers are tiny, however - only 39 EMS calls in a whole country over 5 months. These 39 calls over 5 months were among a section of the population that is over 2 million people. Presenting an increase of 39 calls out of 2 million people as a relative difference of 25% makes this difference seem bigger than it actually is.
In many cases the interpretation is alarmist relative to the limitations of their methods. Language that these findings 'raise concerns regarding vaccine-induced undetected severe cardiovascular side effects' and there is a need to 'promptly investigate potential underlying causes' underrecognize limitations in this study design and are alarmist.
In his email, Robinson had similar concerns about the conclusions drawn from limited information:
Available data from 2.5 million people in Israel found only 2 cases of heart inflammation for every 100,000 people, and most cases were mild. One must also remember that COVID-19 itself can cause heart problems, and the risk for heart problems from COVID-19 is much higher than from vaccination.
The vaccine makers
In a May 4, 2022, email to Lead Stories, Pfizer said it does not typically comment on third-party manuscripts it's not involved in, but provided this general statement:
We take adverse events (AEs) potentially associated with the Pfizer-BioNTech COVID-19 vaccine very seriously. Spontaneously reported AEs are collected for all products to monitor for potential safety issues that may not have been seen in clinical studies. It is important to understand, however, that the AEs reported may not have any causal relationship to the vaccine. Rather, the event may be due to an underlying disease or some other factor such as past medical history or concomitant medication or the AEs may be coincidental.
As with every medical product, Pfizer has robust processes to meet its post authorization responsibilities to closely monitor all AEs and collect relevant information to assess any new potential safety risks that may be associated with the vaccine. In addition to our pharmacovigilance efforts and compliance with various regulatory requirements related to quality and safety, we also cooperate with FDA and regulatory authorities around the world as they independently monitor the safety profile of our vaccine. The U.S. CDC, for example, describes the safety monitoring of the COVID-19 vaccines as 'the most intense safety monitoring in US history.'
Regulatory authorities around the world have authorized the vaccine and expert medical committees have recommended it. With hundreds of millions of people vaccinated with the Pfizer-BioNTech COVID-19 vaccine, the safety profile for the vaccine for all authorized groups continue to be favorable.
Lead Stories also requested a response from Moderna but had not received one at the time of publication.