STORY UPDATED: check for updates below.
Did the Food and Drug Administration (FDA) report that "it is illegal to use anything from nature that is not tested for efficacy and safety"? No, that's not true: The FDA regards herbal supplements as foods, not drugs. Therefore, they are not subjected to the same manufacturing standards to prove they are effective in curing an illness and are ultimately not illegal.
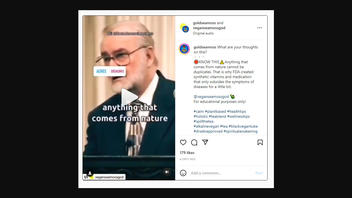
The claim appeared in an Instagram post with a video on November 17, 2022. The in-video text referenced "FDA Approval" of "anything from nature." The caption read:
What are your thoughts on this?
・・・
🛑KNOW THIS⚠️Anything that comes from nature cannot be duplicates. That is why FDA created synthetic vitamins and medication that only subsides the symptoms of diseases for a little bit.@veganseamossgod 🥑
For educational purposes only!#calm #plantbased #healthtips#holistic #teablend #wellnesstips#spillthetea
#alkalinevegan #tea #blackvegantube #drsebiapproved #spiritualawakening #consciousness #lovequotes
This is what the post looked like on Instagram at the time of writing:
(Source: Instagram screenshot taken on Wed Nov 23 18:09:22 2022 UTC)
The man in the reposted Tik Tok video in the post is G. Edward Griffin. He appeared in this June 23, 2009, YouTube video titled, "G. Edward Griffin 3/3, Hijacking of the A.M.A., SBTV 77," where he stated at about the 8:20 mark that "the FDA says it is illegal to use anything from nature that is not tested for efficacy and safety."
The video is posted on a channel known as The Department of Freedom. The description underneath the video reads, "G. Edward Griffin tells the story of the hijacking of the American Medical Association."
Herbal products do not currently have to be tested for efficacy, according to the FDA, because they are considered "foods, not drugs."
An FDA page says that under the Federal Food, Drug, and Cosmetic Act (FD&C Act):
The FDA does NOT have the authority to approve dietary supplements for safety and effectiveness, or to approve their labeling, before the supplements are sold to the public.
Under the FD&C Act, it is the responsibility of dietary supplement companies to ensure their products meet the safety standards for dietary supplements and are not otherwise in violation of the law.
An FDA spokesperson in a November 25, 2022 email confirmed that the above information is accurate stating in part:
Other than the manufacturer's responsibility to meet the safety standards and labeling requirements for dietary supplements and to comply with current good manufacturing regulations, there are no laws or regulations that limit the serving size of a dietary supplement or the amount of a dietary ingredient that can be in a serving of a dietary supplement. This decision is made by the manufacturer and does not require FDA approval.
The proposed Senate bill the Dietary Supplement Listing Act of 2022 would not make it illegal to sell herbal products without efficacy testing or proof, either. It would require purveyors to provide the FDA with a record of "certain claims characterizing the relationship between certain nutrients in the supplement and a disease or a health-related condition."
Lead Stories previously debunked the claim that the measure would ban all non-FDA-approved supplements and ban all herbs.
The links in this Instagram post also mentioned "Dr. Sebi," who died in 2016 and did not have a license to practice medicine. Other Lead Stories fact checks on Dr. Sebi can be found here.
Updates:
-
2022-11-28T21:34:15Z 2022-11-28T21:34:15Z Updated with November 25, 2022, response from FDA spokesperson.