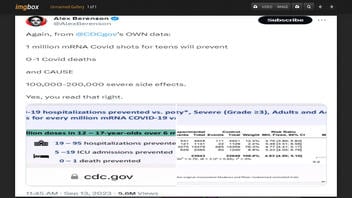
Does data from a Centers for Disease Control and Prevention (CDC) briefing from September 2023 show that mRNA COVID vaccines "CAUSE 100,000-200,000 severe side effects" for every one million shots given to "teens"? Yes, that's the label on a data chart, but it's misleading wordplay that makes vaccine aches and pains sound scarier than they are. The Food and Drug Administration's definition of what constitutes a "severe" side effect may not align with a layperson's understanding of the word. An infectious disease expert told Lead Stories that FDA-rated "severe" side effects "have no significant or lasting health consequences." The CDC's Immunization Safety Office said less than 1 percent of people ages 12-17 who received the Pfizer-BioNTech booster dose mRNA COVID-19 vaccine received medical attention after vaccination.
The claim appeared in a post (archived here) published on X, the social media platform formerly known as Twitter, by writer and former New York Times reporter Alex Berenson on September 13, 2023. The post's caption says:
Again, from @CDCgov's OWN data:
1 million mRNA Covid shots for teens will prevent
0-1 Covid deaths
and CAUSE 100,000-200,000 severe side effects.
Yes, you read that right.
This is what the post looked like on Twitter at the time of writing:
(Source: Twitter screenshot taken on Thu Sep 21 15:07:59 2023 UTC)
The information reported by Berenson in the social media post comes from a slide presentation (archived here) given to a September 12, 2023, meeting of the Advisory Committee for Immunization Practices (ACIP) titled "Evidence to Recommendations Framework: 2023 - 2024 (Monovalent, XBB Containing) COVID-19 Vaccine." ACIP develops recommendations on the use of vaccines in the United States. At this particular meeting, ACIP endorsed updated COVID-19 vaccines for the fall of 2023, which the CDC accepted (archived here).
Dr. James Lawler with the Division of Infectious Diseases at the University of Nebraska Medical Center told Lead Stories in a September 22, 2023, email that he agrees with Berenson's post up to a point. He said;
Mr. Berenson appears to interpret the numbers in the same way I would, assuming that it shows slightly more than 10% increase in risk for a Grade 3 reaction for subjects receiving the mRNA COVID vaccine vs. those receiving placebo. Unfortunately, this is where the similarity in our analysis ends, because Mr. Berenson then intentionally misleads his audience regarding the meaning of those data and deliberately excludes important context for how it fits into a balance of risk and benefit.
The FDA defines a Grade 3 reaction as "severe," which is what Berenson is referring to when he says, "severe side effects."
Lawler said Berenson, a Random House-published author, is using "the terminology of 'severe' with a clear intent to sow fear and doubt about vaccines." He continued:
[I]t is easy to see how people would be alarmed without the appropriate explanation, which he conveniently omits. It is important to look at how the FDA and industry define a 'severe adverse event' ... A 'severe' adverse event sounds really bad, but it simply defines a reaction that is more than mildly irritating. It does not indicate that the person needed to seek acute healthcare. Hospitalization or a visit to the ER is what defines a "Grade 4" adverse event.
The FDA defines a Grade 4 reaction as a "potentially life-threatening event." Grade 5 is death. Those levels of reactions are extremely rare. The data the CDC slide presentation was taken from shows less than 0.1 percent of the initial clinical trial participants for the Moderna mRNA COVID-19 vaccine had a Grade 4 reaction.
Because of that, Lawler said, nearly all of the counted reactions cited by Berenson in his post are Grade 3. While they are "severe" by definition, Lawler added, "they have no significant or lasting health consequences." He said:
Grade 3 events span a wide range, including an area of swelling or redness of 4 inches or more at the injection site, somebody taking a prescription pain medication, or discomfort at rest. For systemic reactions, a temperature over 102, a high heart rate or blood pressure, headache, fatigue, or muscle aches interfering with daily activity all count, among others.
Lawler said he had his own personal experience with what would be considered a "severe" side effect:
When I received my second dose of the mRNA vaccine, I had a mild headache and fatigue, so I took a 2-hour nap at 5 or 6 in the evening, something I never do. By the next morning, I felt fine - but this technically would have counted as a Grade 3 event, because it interfered with my normal daily activity.
Centers for Disease Control and Prevention
The CDC has observed similar safety figures with the mRNA COVID-19 vaccines since they became available in late 2020. A September 22, 2023, email from the agency's Immunization Safety Office said (emphasis theirs):
What we've observed after 2.8 million doses of Pfizer-BioNTech booster dose mRNA COVID-19 vaccination among people ages 12-17 years is that <1% received medical attention after vaccination - far less than what this post claims. For more information, see this link: Safety Monitoring of COVID-19 Vaccine Booster Doses Among Persons Aged 12-17 Years -- United States, December 9, 2021-February 20, 2022 | MMWR (cdc.gov)
The same was also true after over 8.9 million people ages 12-17 years received their primary series mRNA COVID-19 vaccination: <1% received medical attention after vaccination. COVID-19 Vaccine Safety in Adolescents Aged 12-17 Years -- United States, December 14, 2020-July 16, 2021 | MMWR (cdc.gov)
Other benefits
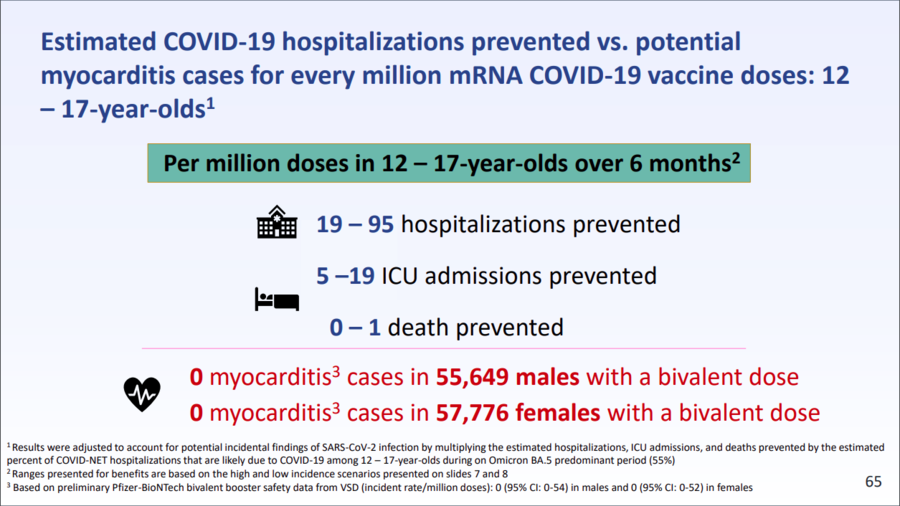
Slide 65 of the CDC presentation, which is one of two featured in the social media post by Berenson, struck Lawler as particularly misleading when taken in isolation. The slide appears below:
(Source: CDC slide 65 from Mon Sep 25 2023 UTC)
Lawler called the information highlighted from this slide the "real kicker," because with it, he said, Berenson suggests that "additional vaccination of teens would prevent only one death and fewer than 100 hospitalizations among teens" for every million vaccinated. Lawler said the benefits are much broader:
What is not discussed is the large toll of long COVID and post-COVID health effects that vaccines can prevent. By the CDC's very restrictive definition of symptoms lasting 3 months or more, about 2% of adolescents or teens (ages 12-17) have experienced long COVID and almost 1% are still CURRENTLY experiencing long COVID. ... Two percent of American teens (age 12-17) roughly equates to 540,000 who have experience post-COVID symptoms for more than 3 months. Long COVID can have significant impacts on physical fitness and performance, sleep, cognitive function and concentration, and social interaction. Based on the average of multiple studies that have looked into vaccine effectiveness at preventing long COVID vaccination may reduce the risk of long COVID by 40%. That would translate to more than 200,000 teens who could have had their long COVID prevented by vaccination. I think if you asked teens and parents if they would rather have annoying symptoms for a day or potentially debilitating consequences of COVID infection lasting 3 months or more, the answer would be pretty easy.
The above risk-benefit calculation doesn't even address the protective impact vaccination has against transmission in households and communities. Undervaccinated kids and teens result in higher rates of secondary transmission of COVID in households and across the community. This significantly increases the risk of infecting older adults and others at highest risk for severe infection and death.
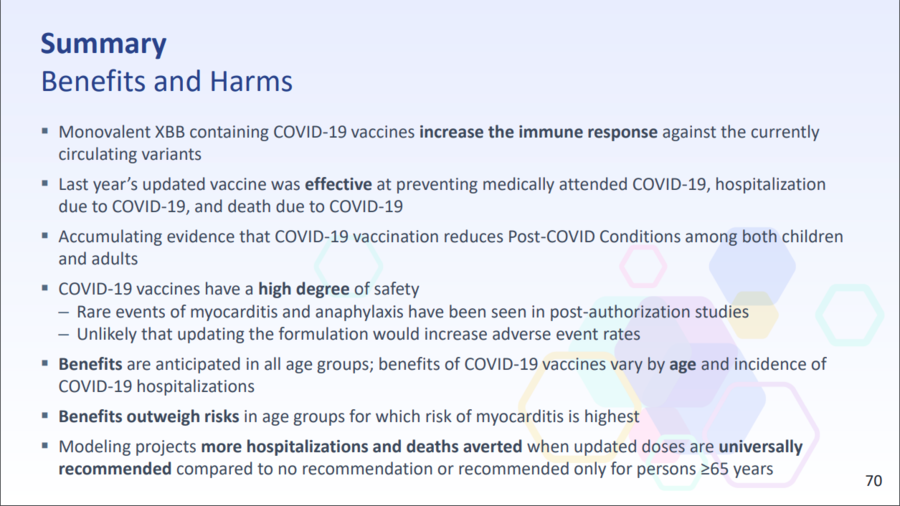
In its email response to Lead Stories, the CDC noted that slide 70 of the presentation more clearly illustrates the pros and cons of getting the updated COVID-19 vaccine for fall 2023:
(Source: CDC slide 70 from Mon Sep 25 2023 UTC)
In the conclusion of his email to Lead Stories, Lawler added:
No vaccine or medication is completely without risk. But the data on COVID vaccines is clear and overwhelming. They have great safety record, with low rates of truly concerning side-effects after billions of doses administered. They also have a profound impact in reducing the spread of COVID and the consequences of COVID - across all age groups. Fear-mongering about vaccines gets people social media and political points, but it results in real damage to real people.
Read more
Additional Lead Stories fact checks of claims related to vaccines can be found here.