Did controversial wellness coach Barbara O'Neill endorse a brand of CBD gummies as a remedy for high blood pressure in a social media post? No, that's not true: O'Neill, who is "permanently prohibited from providing any health services" in Australia, is the subject of a fake article designed to resemble content from the Med Journal Research & Practical Medical Advice website. Instead, it is a fabricated pitch using a person known in alternative medicine circles to promote a product making unproven claims.
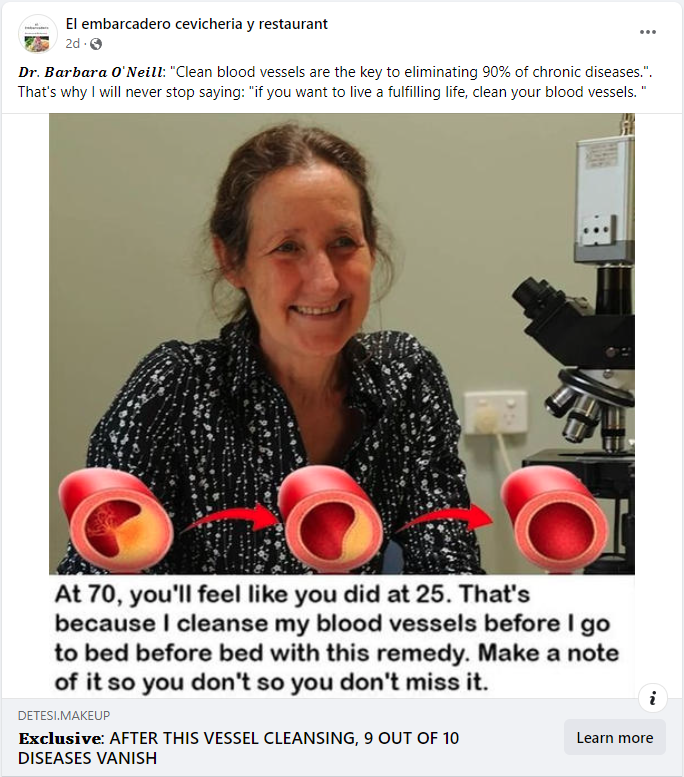
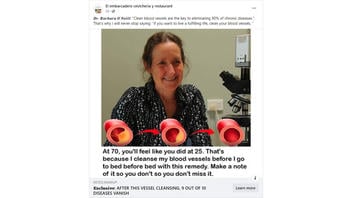
The claim appeared in a post (archived here) on Facebook by El embarcadero cevicheria y restaurant on January 29, 2024. The captions surrounding the post said:
𝑫𝒓. 𝑩𝒂𝒓𝒃𝒂𝒓𝒂 𝑶'𝑵𝒆𝒊𝒍𝒍: 'Clean blood vessels are the key to eliminating 90% of chronic diseases.'. That's why I will never stop saying: 'if you want to live a fulfilling life, clean your blood vessels. '
At 70, you'll feel like you did at 25. that's because I cleanse my blood vessels before I go to bed before bed with this remedy. Make a note of it so you don't so you don't miss it.
This is what the post looked like on Facebook at the time of writing:
(Source: Facebook screenshot taken on Wed Jan 31 16:33:13 2024 UTC)
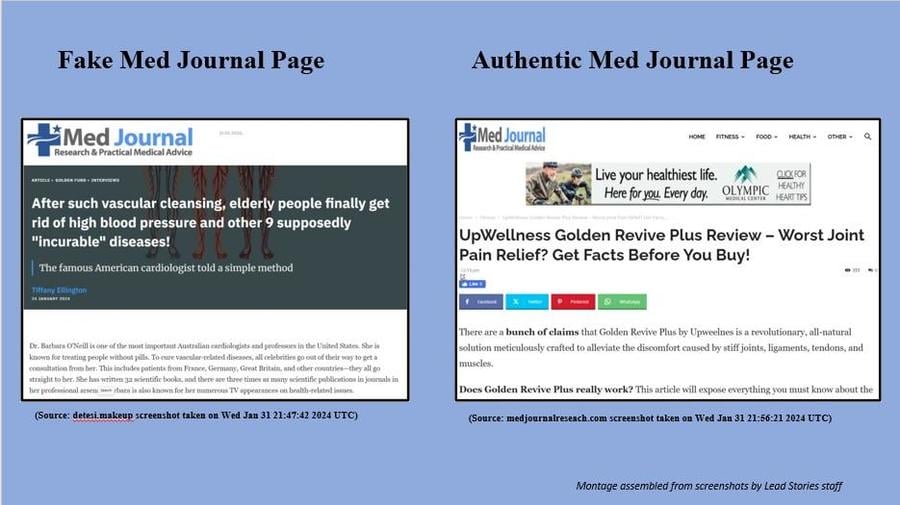
The post on Facebook links to what looks like an article (archived here) from the Med Journal Research & Practical Medical Advice website, with the title "After such vascular cleansing, elderly people finally get rid of high blood pressure and other 9 supposedly 'incurable' diseases!" But despite generally matching the website's appearance, it features a different web address under a different domain name. Additionally, there are no articles featuring "Barbara O'Neill" on the Med Journal website.
Web addresses and websites
Here are the two addresses for comparison:
- Facebook post link to the fake article: https://detesi.makeup/bbla/?fbclid=IwAR3MxGQi-bxV139WQOfflqc-XQhIzAVFFR4O5FfUJsapuisDQWP90IUdkT0
- Med Journal website link: https://medjournalresearch.com/upwellness-golden-revive-plus-review-joint-pain-relief/
As you can see below, both screenshots are similar and incorporate the Med Journal logo in the top left-hand corner of the page, but the fake page contains no links to the rest of the Med Journal website, which is standard on Med Journal's site.
No Barbara
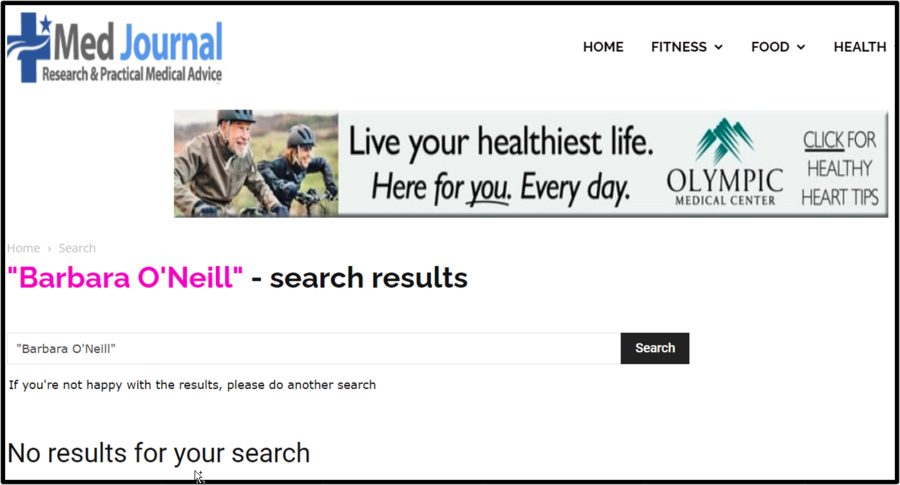
To double-check our suspicions, Lead Stories searched for "Barbara O'Neill" on the Med Journal website (archived here) to see if there was a story matching the one on the fake website page and the answer was "No results for your search." You can see it below:
(Source: Med Journal screenshot taken on Wed Jan 31 22:10:08 2024 UTC)
Not a medical journal
Although it carries the moniker Med Journal Research & Practical Medical Advice, the website is not a real medical journal detailing medical research. Its "About Us" section at the bottom of the page spells out its mission, which is to ultimately sell things:
Med Journal Research content is strictly informational and should not be considered medical advice. See a certified medical professional for diagnosis and treatment recommendations.
... MedJournalResearch.com is a product review website which aims to introduce some much-needed honesty and transparency to the world of online reviews.
At MedJournalResearch.com, visitors will find detailed reviews of popular products currently available online. Those products come from categories like nutritional supplements, financial products, and online business ideas.
O'Neill is well-known in the alternative medicine community, but was sanctioned by Australia's Health Care Complaints Commission (HCCC) in 2019. A statement from the HCCC, following an investigation, said:
The Commission is satisfied that Mrs O'Neill poses a risk to the health or safety of members of the public. The Commission therefore makes the following prohibition order:
- Mrs O'Neill is permanently prohibited from providing any health services, as defined in s4 of the Health Care Complaints Act 1993 (the Act), whether in a paid or voluntary capacity.
CBD claims
The fake article with the false O'Neill endorsement eventually links to a website selling CBD gummies (archived here) that claims they regulate blood pressure, balance blood sugar, lower bad cholesterol, increase good cholesterol and reverse insulin resistance.
In a January 31, 2024, email to Lead Stories, the U.S. Food and Drug Administration (FDA) said Epidiolex, which is used to treat certain kinds of seizures, is the only FDA-approved CBD drug product. The FDA Office of Media Affairs continued:
We are aware that some firms are marketing CBD products to treat diseases or for other therapeutic uses, and we have issued several warning letters to such firms. Under the FD&C Act, any product intended to have a therapeutic or medical use, and any product (other than a food) that is intended to affect the structure or function of the body of humans or animals, is a drug. Drugs must generally either receive premarket approval by FDA through the New Drug Application (NDA) process or conform to a 'monograph' for a particular drug category, as established by FDA's Over-the-Counter (OTC) Drug Review. CBD was not an ingredient considered under the OTC drug review. An unapproved new drug cannot be distributed or sold in interstate commerce.
Additional FDA information on CBD products is available here.
Read more
Additional Lead Stories fact checks of claims related to O'Neill can be found here.