STORY UPDATED: check for updates below.
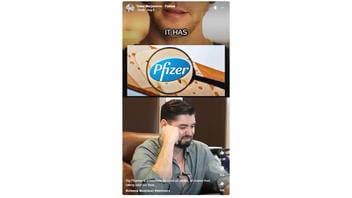
Will bioengineered rennet, an enzyme necessary for cheese production and made primarily by Pfizer, make you sick, as a video on Facebook implies? No, that's not true: Rennet is not considered genetically modified and is safe for human health, several experts told Lead Stories. Rennet is used to generate the final cheese product, but is not an ingredient in cheese, the Food and Drug Administration confirmed to Lead Stories.
A version of the claim appeared in a reel shared on Facebook on August 7, 2024, (archived here) with a caption that read:
Big Pharma is a business focused on profits, of course their taking over our food.
#cheese #eatclean #wellness
Here is how the video appeared at the time of writing:
(Source: Facebook screenshot taken Wed Aug 14 08:57:00 2024 UTC)
The reel suggests that bioengineered rennet, known as fermentation-produced chymosin (FPC), an enzyme used to produce cheese, harms humans. The video claims it is used because "they" -- presumably, pharmaceutical companies -- "want to make you sick."
According to three independent experts with whom Lead Stories spoke, such insinuations are false.
Chymosin rennet is a catalyst for creating cheese from milk
Rennet is a set of naturally occurring enzymes found in the lining of the fourth stomach of ruminant animals, such as goats, lambs and calves, according to the 2022 book "Value-Addition in Food Products and Processing Through Enzyme Technology" (archived here). The passage is visible here (archived here). Chymosim, rennet's key component, is an enzyme that curdles the casein protein in milk to ultimately create cheese products. Rennet clots milk to separate liquid from solids and is a catalyst in the cheese-making process, but is not considered an ingredient in cheese.
There are four types of rennet: microbial, vegetable, animal and fermentation-produced chymosin (FPC). As its name suggests, "fermentation produced" refers to how that type of rennet is made.
Melissa Prest (archived here), a Chicago-based registered dietician nutritionist and spokesperson for the Academy of Nutrition and Dietetics (archived here), a trade organization, told Lead Stories that bioengineered rennet received approval (archived here) from the Food and Drug Administration (FDA) in the 1990s. Prest wrote in an email received on August 16, 2023:
FPC was produced in the 1980s after scientists learned how to transfer a gene from bovine cells into microbes, enabling them to produce chymosin. Those microbes underwent a fermentation process to create fermentation-produced chymosin (FPC). Chymosin and rennet are enzymes used to make cheese, and based on how the cheese is made, it will contain one of these enzymes, but not necessarily both.
Industry shift from naturally occurring to fermented chymosin
As appetites for cheese increased and veal consumption decreased in recent decades, cheese makers have turned to producing rennet synthetically to keep up with demand, according to Kevin Folta (archived here), a professor of horticultural science at the University of Florida.
"Chymosin was one of the first genetically engineered compounds in a microbe [used] for cheese production," Folta told Lead Stories in a phone interview on August 15, 2024. It is created using an "optimized system" to generate recombinant-based or genetically cloned (archived here) proteins.
"Rennet is not an ingredient in the cheese. It's probably there in some trace amounts, but the idea is that it's used to do the reaction and is not included in the final product," Folta said, adding that there is "no associated risk with the recombinant protein."
In an email received on August 26, 2024, the FDA confirmed these sentiments, writing that the FPC process is not used to make cheese:
The FPC process is used to make chymosin, which is purified, and the purified chymosin is used in the cheese making process. Use of rennet or chymosin in the cheese making process can result in residual amounts of these substances in the final cheese product.
FPC is not categorized as a genetically modified organism (GMO)
Jon Entine (archived here), executive director of the nonprofit organization Genetic Literacy Project (archived here), a nonprofit that combats misinformation and disinformation about science, told Lead Stories in a phone interview on August 15, 2024, that FPC is neither genetically modified nor considered dangerous to human health.
"Rennet is not a GMO but is the product of a GMO process," he said.
Entine referred Lead Stories to an FAQ page (archived here) written by the Genetic Literacy Project, which describes the GMO process as:
Ninety percent of cheeses and almost all hard cheeses are produced with an enzyme called chymosin that is generated by genetically modified bacteria, known as fermentation-produced chymosin, or FPC. For many years, milk in the United States was produced by cows treated with rBST, a naturally occurring hormone researchers began producing in genetically engineered microbes in 1985 ...
The final products made from these substances usually contain no genetically engineered material by the time they reach grocery store shelves. Food safety regulators say that these products are substantially equivalent to foods made without the assistance of genetic engineering.
Following our interview, the Genetic Literacy Project published on August 16, 2024 an explainer (archived here) regarding health concerns related to FPC. In it, Entine specifically addressed the video on Facebook, noting that it "makes numerous false claims," including that "foods through GM can be less safe" and that "rennet produced using GM was barely tested."
"GM is a process of modification" and not a product, Entine told Lead Stories.
No known risk associated with cheese made from FPC
The FDA designates FPC as "Generally Regarded as Safe" (GRAS) (archived here). This group includes a variety of substances, from commonly used flavorings and spices to phosphates and the food additive carrageenan that are considered harmless under their prescribed uses.
"Specifically, the information must demonstrate that there is a reasonable certainty of no harm to consumers when an ingredient is proposed or intended for use in food," wrote an FDA spokesperson in an email received on August 26, 2024.
The FDA also sent Lead Stories the related GRAS documents (archived here) and the data evaluated by the FDA. The agency also sent Lead Stories reports documenting the safety evaluation, which we archived here and here.
"The enzyme is chymosin, the same exact enzyme that comes out of the animal. When [producers] call it 'generally regarded as safe,' it's because it can't be anything but. It comes from the animal's gene, just expressed in a big vat fermenter rather than having to harvest it out of a gut pile," Folta said.
Other Lead Stories health-related fact checks can be read here.
Updates:
-
2024-08-27T16:57:58Z 2024-08-27T16:57:58Z Adds comment, details from FDA.