Were embryonic stem cells used in the development of Regeneron, the antibody treatment given to President Donald Trump? No, that's not true: Regeneron Pharmaceuticals, the company behind the antibody cocktail, told Lead Stories that it did not use such cells in the development of its therapy. However, the treatment was tested using cells that were originally derived from an aborted fetus.
The claim appeared in a post (archived here) on Facebook on October 7, 2020. It opened:
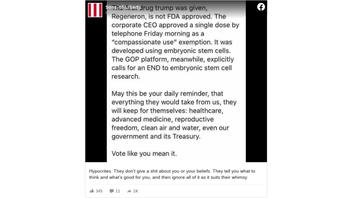
The first drug trump was given, Regeneron, is not FDA approved. The corporate CEO approved a single dose by telephone Friday morning as a "compassionate use" exemption. It was developed using embryonic stem cells.
This is what the post looked like at the time of writing:
(Source: Facebook screenshot taken on Fri Oct 9 16:39:42 2020 UTC)
Last week, the White House announced that Trump, who tested positive for COVID-19, had received an experimental antibody cocktail manufactured by Regeneron Pharmaceuticals. You can read what the company says about REGN-COV2 here. In an email to Lead Stories, Alexandra Bowie, a spokeswoman for Regeneron, said that the antibody cocktail was not developed using stem cells. She wrote:
We did not use human stem cells or human embryonic stem cells in the development of REGN-COV2.
However, the treatment was tested in cells that were originally derived from a human fetus. Here's more from Bowie:
We did use the HEK293T cell line to test our antibodies' ability to neutralize the SARS-CoV-2 virus (they were used to make 'pseudovirus' that looks like the Spike protein). HEK293s are considered 'immortalized' cells (not stem cells) and are a common and widespread tool in research labs. This cell line was originally derived by adenovirus transformation of human embryonic kidney cells in 1977. After this, it was further transformed at Stanford in the '80s with SV40 T-antigen (hence the 'T'). HEK293T wasn't used in any other way, and fetal tissue was not used in this research.
Back in April, before Regeneron made international headlines, the company released a statement detailing its position on stem cell research. It read, in part:
As is the case with many other science-focused biotechnology companies, Regeneron uses a wide variety of research tools and technologies to help discover and develop new therapeutics. Stem cells are one such tool. The stem cells most commonly used at Regeneron are mouse embryonic stem cells and human blood stem cells. Currently, there are limited research efforts employing human-induced pluripotent stem cell lines derived from adult human cells and human embryonic stem cells that are approved for research use by the National Institutes of Health and created solely through in vitro fertilization. Research using such stem cells allows Regeneron to model complex diseases, test new drug candidates and can help unlock new scientific insights that ultimately could lead to the discovery of new treatments for people with serious diseases.
The Trump administration and Republicans, generally, are against medical research using fetal tissue from recent abortions and have taken steps to curtail it.