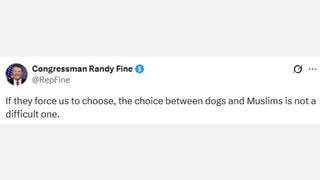
FDA WILL NOT AUTHORIZE OR APPROVE ANY COVID-19 VACCINE
This is what the post looked like on Instagram on April 29, 2021:
(Source: Instagram screenshot taken on Thu Apr 29 21:22:40 2021 UTC)
The clip of Hahn speaking is from "COVID-19: An Update on the Federal Response," from a September 23, 2020, hearing by the Senate Committee on Health, Education, Labor & Pensions. The Instagram clip is a portion of Hahn's testimony that followed his explanation of the FDA's emergency use authorization (EUA) process. The entirety of Hahn's statement in the Instagram clip was:
In the end, FDA will not authorize or approve a vaccine that we would not feel comfortable giving to our families. On behalf of the 17,000+ employees of the FDA, I want to make the following commitments today to the American public and this committee:
FDA will not authorize or approve any COVID-19 vaccine before it has met the agency's rigorous expectations for safety and effectiveness. Decisions to authorize or approve any such vaccine or therapeutic will be made by the dedicated career staff at FDA through our thorough review processes and science will guide our decisions. FDA will not permit any pressure from anyone to change that.
I will fight for science, Mr. Chairman, I will fight for the integrity of the agency, and I will put the interests of the American people before anything else. Thank you and I look forward to answering your questions.
The full video of Hahn's testimony can be found on the committee's website. The start of Hahn's entire testimony begins at the 1 hour, 2 minute and 53 second mark; the portion of his testimony used in the Instagram clip begins at the 1 hour, 7 minute and 58 second mark.
The FDA considers using EUA when there is a widespread public health emergency that could be lessened by the use of a medical product. As of April 29, 2021, the agency authorized EUA for the Pfizer-BioNTech, Moderna and Janssen (Johnson & Johnson) COVID-19 vaccines. The path to EUA approval for a COVID-19 vaccine is detailed on the FDA's website. The process includes the use of clinical trials and approval from various committees composed of health officials.
Lead Stories has previously debunked several false claims about COVID-19 and the vaccines used to combat the disease. We have also previously reported that some of the COVID-19 vaccines used in the U.S. have received emergency use authorization from the FDA.