Does hydroxychloroquine work as a vaccine against COVID-19? No, that's not true: The Food & Drug Administration and the World Health Organization don't recommend the drug, which is used to treat malaria, lupus and rheumatoid arthritis, for the treatment of COVID.
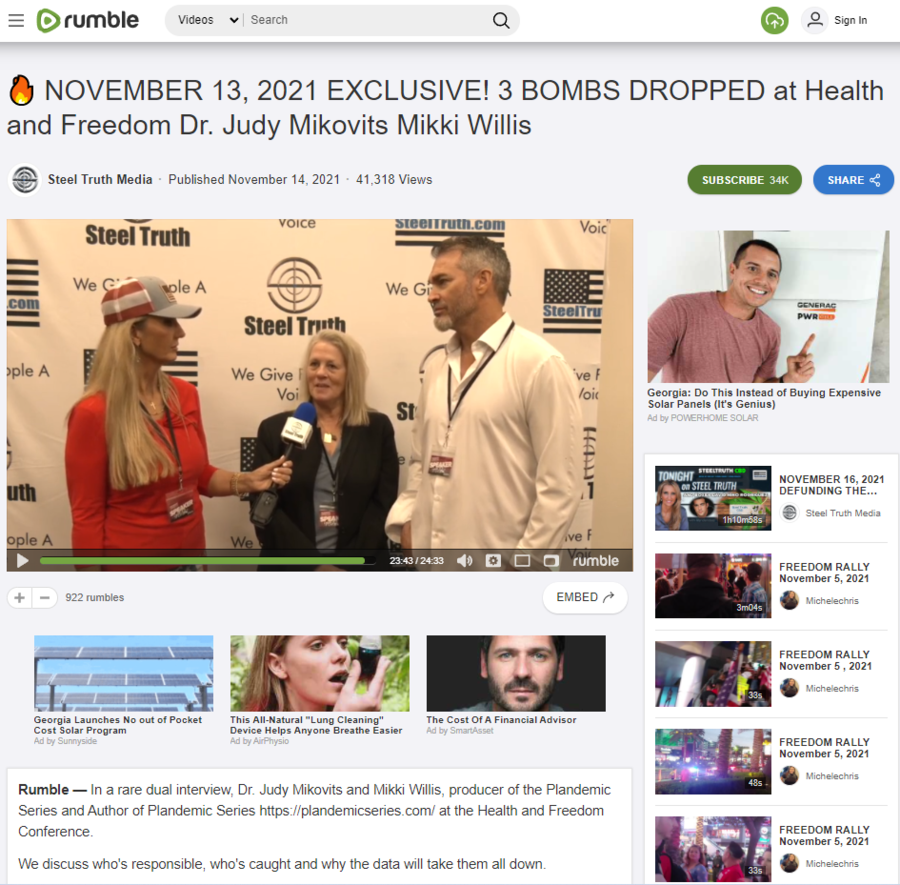
The claim appears in a video posted to Rumble (archived here) on November 14, 2021, titled "NOVEMBER 13, 2021 EXCLUSIVE! 3 BOMBS DROPPED at Health and Freedom Dr. Judy Mikovits Mikki Willis." It opens:
In a rare dual interview, Dr. Judy Mikovits and Mikki Willis, producer of the Plandemic Series and Author of Plandemic Series https://plandemicseries.com/ at the Health and Freedom Conference.
We discuss who's responsible, who's caught and why the data will take them all down.
This is what the post looked like on Rumble on November 17, 2021:
(Source: Rumble screenshot taken on Wed Nov 17 18:13:07 2021 UTC)
On the Rumble video, Mikovits, a Ph.D. researcher whose COVID misinformation has been debunked multiple times by Lead Stories, claims hydroxychloroquine provides immunity to COVID. Her stance is based on a rephrasing of the definition of "vaccine."
In September 2021, the Centers for Disease Control and Prevention modified its definition of the words "vaccine" and "vaccination" on its website. Before it was changed, the definition for "vaccination" said, "the act of introducing a vaccine into the body to produce immunity to a specific disease." After the switch, the word "immunity" was changed to "protection."
At 2:00 in the video, Mikovits says:
The CDC even changed, just a week or so ago, the definition now is not immunity, but protection from COVID.
Then at 2:25, she uses that change in definition to support her claim that hydroxychloroquine works as a vaccine against the virus:
For those people who had COVID, they're immune and they exceed the CDC requirement. And then we have them certifying under penalty of perjury in a legal affidavit that they take the appropriate, every monthly booster, every 21-day booster of an oral vaccination which would be hydroxychloroquine, which was published in 2005 to be a vaccine, not only for SARS (severe acute respiratory syndrome), but for HIV.
It's uncertain what Mikovits is referring to when she talks about the "CDC requirement" for immunity. The term "threshold of protection" is used when antibodies increase to a level that provides effective protection following infection or immunization for a disease. Hydroxychloroquine is medicine and does not produce antibodies.
The study she refers to in the interview was published on August 22, 2005, under the title, "Chloroquine is a potent inhibitor of SARS coronavirus infection and spread." It was tested as a treatment and showed some promise in primates, but wasn't tested on humans. SARS, also known as SARS-CoV, is not the same virus as COVID-19, which has a similar name, SARS-CoV-2.
Later in the interview, Mikovits continues to push the notion that the drug will build immunity in people. At 15:45, she says:
If you're using hydroxychloroquine, you're boosting it. ... You'll truly be immunized and you will exceed CDC's new guideline, because they changed the definition knowing what we're saying now. ... So, you will have protection and you won't need anything. You've got immunity.
Under pressure from then-President Donald Trump in the early days of the pandemic, the FDA responded by looking into hydroxychloroquine as a therapeutic drug.
The FDA first granted emergency approval for hydroxychloroquine as a COVID treatment in March 2020, but then reversed that permission on June 15, 2020, after investigators in a nationwide clinical trial found evidence it caused serious heart problems. The FDA announced:
... chloroquine and hydroxychloroquine are unlikely to be effective in treating COVID-19 for the authorized uses in the EUA [emergency use authorization]. Additionally, in light of ongoing serious cardiac adverse events and other potential serious side effects, the known and potential benefits of chloroquine and hydroxychloroquine no longer outweigh the known and potential risks for the authorized use.
In a study published in the December 2020 Journal of the American Medical Association, the leaders of a nationwide clinical trial wrote:
Hydroxychloroquine has been widely promoted as a potential therapy for COVID-19 due to its anti-inflammatory effects and in vitro studies suggesting antiviral activity ... Among adults hospitalized with respiratory illness from COVID-19, treatment with hydroxychloroquine, compared with placebo, did not significantly improve clinical status at day 14.