Are the injections against COVID-19 "deadly experimental shots" and not vaccines? No, that's not true: Medical science classifies all three injected preventatives as vaccines and former President Donald Trump, whose Operation Warp Speed set records in vaccine development, has repeatedly urged the vaccine-hesitant to get the shot as he did, calling it a vaccine. The three vaccines in use in the U.S. were tested on tens of thousands of volunteer patients and found safe and effective, a determination vouched for in science journal articles reviewed by independent experts, some of whom were likely jealous competitors who nonetheless checked and agree with the lead scientists' findings. The Food and Drug Administration reviewed the test results, carried out its own analysis of the ingredients and the manufacturing methods and approved the first two vaccines for Emergency Use Authorization (EUA) in 2020, a year when 375,000 died of COVID-19. In 2021, a year in which about 400,000 died of COVID-19, Janssen received EUA for its vaccine and Pfizer applied for and was granted the long-term license for its vaccine.
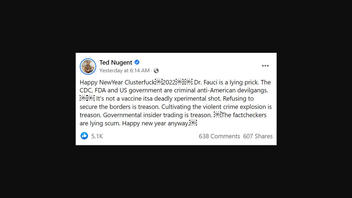
The claim appears in a January 3, 2022, Facebook post (archived here) published under the title "Happy NewYear Clusterfuck2022!" It opened:
Dr. Fauci is a lying prick. The CDC, FDA and US government are criminal anti-American devilgangs. It's not a vaccine itsa deadly xperimental shot. Refusing to secure the borders is treason. Cultivating the violent crime explosion is treason. Governmental insider trading is treason. The factcheckers are lying scum. Happy new year anyway
This is what the post looked like on Facebook at the time of writing:
(Source: Facebook screenshot taken on Tue Jan 4 21:26:31 2022 UTC)
The Facebook post makes several statements of opinion, which are outside the scope of fact checking. This fact check only addresses the false claim that the vaccines aren't vaccines and that they are deadly.
The vaccines are vaccines
Lead Stories has collected here the findings of its multiple investigations into claims about the definition of vaccine, changes to the term that match changes in technology and other attempts to discredit the public health community's war on the virus that causes COVID. Our finding: Using needles or sprays to trigger immune responses that protect us against viruses when they show up is vaccination and that's what the three U.S.-approved shots do.
The "experimental" is long over
Professional medical scientists peer-reviewed the safety testing for publication of results in a top journal of medical science.
The results of the Pfizer safety trial, which enrolled more than 40,000 volunteers, were published in a December 10, 2020, article in the New England Journal of Medicine.
The results of the Moderna trial, which enrolled more than 30,000 volunteers, were published in a December 30, 2020, New England Journal of Medicine article.
Results of the Janssen vaccine safety trial, which enrolled more than 19,000 volunteers, were published in an April 21, 2021, article in the New England Journal of Medicine.
Peer review is the process by which a scientist's work is sent to independent experts who review the methods, the data and the conclusions, looking for errors and omissions and suggesting changes that -- in the worst case -- cause a paper to be rejected and -- in the best case -- strengthen the final product before it is published. Further peer review happens after publication, when even more peers are able to see the work and attack weaknesses. None of the three papers has been retracted.
FDA Emergency Use Authorization is not passive, and FDA rated all three vaccines safe and effective
The FDA issued Emergency Use Authorization for the Pfizer-BioNTech vaccine on December 11, 2020. One week later, the FDA granted the same permit for the Moderna COVID-19 vaccine. Both of them use mRNA technology to train the human immune system to attack antibodies with the spike protein that is characteristic of the SARS-CoV-2 virus that causes COVID-19 disease. On February 27, 2021, the FDA granted an EUA for the Janssen vaccine, which uses a modified piece of the SARS-CoV-2 virus to train the immune system to attack SARS-CoV-2 if a person becomes infected.
Here's how the FDA describes the EUA process:
In certain types of emergencies, the FDA can issue an emergency use authorization, or EUA, to provide more timely access to critical medical products (including medicines and tests) that may help during the emergency when there are no adequate, approved, and available alternative options.
The EUA process is different than FDA approval, clearance, or licensing because the EUA standard may permit authorization based on significantly less data than would be required for approval, clearance, or licensing by the FDA. This enables the FDA to authorize the emergency use of medical products that meet the criteria within weeks rather than months to years.
There is no competitively reviewed, scientifically valid evidence the vaccines are "deadly"
No scientific institution or researcher that has withstood competitive review has declared the vaccines deadly. Lead Stories had investigated, as of Jan. 4, 2022, dozens of separate claims of vaccine deadliness, finding no credible basis to the claims and multiple instances of innumeracy, fakery, mis-statement of clearly labelled data and oversimplification of complex data sets. A partial collection of Lead Stories' investigations is archived here.
There have been documented cases of rare injury or death from the vaccines, but the FDA and multiple independent medical professionals have found the vaccines are overwhelmingly safer than rising COVID-19 disease. The CDC's guidance is based on the hundreds of millions of people who have been safely vaccinated.