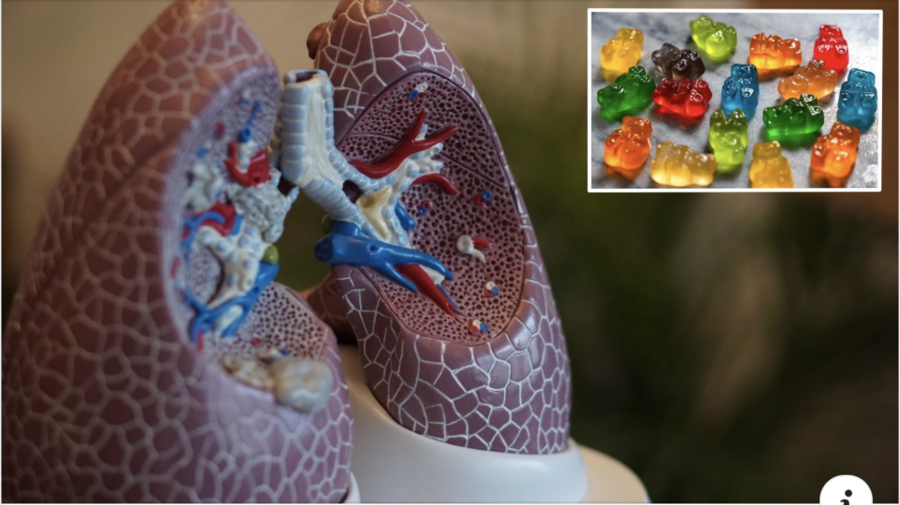
STORY UPDATED: check for updates below.

Are "gummies" approved by the FDA as clinical treatment for quitting smoking? No, that's not true: The Food and Drug Administration does not include gummies on their list of approved smoking cessation products. It has approved nicotine gum, which is chewing gum but that is not a candy gummy.
The claim appeared as a Facebook post (archived here) on January 4, 2022, under the title "Hard To Find 'Quit Smoking' Gummies Actually Work." It opened:
It's official, these gummies are approved by the FDA as a clinical treatment for quitting smoking. Say goodbye to COPD with 2 gummies/day!
This is what the post looked like on Facebook at the time of writing:
(Source: Facebook screenshot taken on Fri Jan 7 22:15:53 2022 UTC)
The image on the Facebook post is a mashup of two stock photos that can be found here. The post claims to link to an article titled, "Hard To Find 'Quit Smoking' Gummies Actually Work." However, the link goes to the website The Best Therapize with a post titled, "All You Need to Know About Essential Oils: A Comprehensive Guide to Natural Remedies," which has nothing to do with candy gummy bears or smoking.
The FDA list of approved products that help people quit smoking does not include candy gummies. It states:
But the FDA has approved several smoking cessation products designed to help users gradually withdraw from smoking (that is, 'wean' themselves from smoking) by using specific amounts of nicotine that decrease over time. This type of product is called a 'nicotine replacement therapy' or NRT. It supplies nicotine in controlled amounts while sparing you from other chemicals found in tobacco products.
None of the approved products are candy gummy bears:
Over-the-counter NRTs are approved for sale to people age 18 and older. They are available under various brand names and sometimes as generic products. They include:
Skin patches (also called 'transdermal nicotine patches'). These patches are placed on the skin, similar to how you would apply an adhesive bandage.
Chewing gum (also called 'nicotine gum'). This gum must be chewed according to the labeled instructions to be effective.
Lozenges (also called 'nicotine lozenges'). You use these products by dissolving them in your mouth.
The Facebook post also states, "Say goodbye to COPD with 2 gummies/day!" As Lead Stories previously reported, neither the FDA nor the American Lung Association include gumdrop-form medications on their list of tested and approved medications for treatment of the condition, whose full name is Chronic Obstructive Pulmonary Disease.
The FDA responded on January 10, 2022, to Lead Stories' comment request by directing to their website article titled, 'Fact or Fiction: What to Know About Smoking Cessation and Medications." This noted the medicines that are approved by the FDA to help quit smoking, and gummies were not included in the list:
All smoking cessation medications are the same.
FICTION!
There are 3 different types of medications approved by the FDA to help you quit smoking -- nicotine replacement therapies, bupropion, and varenicline. Nicotine replacement therapy medications are available in five different forms: inhaler, nasal spray, patch, gum, or lozenge (see this FDA Consumer Update for more information). The medications vary in how they affect the body, how they are used, and how long they should be used. Some are available over-the-counter, and others require a prescription from your doctor. Talk to your doctor to determine which option might be best for you.
Updates:
-
2022-01-10T18:04:55Z 2022-01-10T18:04:55Z This story was updated January 10, 2022, with a response from the FDA.