Does the childhood vaccine Vaxelis contain "toxic" levels of formaldehyde and aluminum? No, that's not true: While the six-in-one vaccine does contain trace amounts of formaldehyde, the Centers for Disease Control and Prevention lists formaldehyde as a "residual inactivating ingredient" that is used to kill viruses or inactivate toxins during the manufacturing process. Aluminum salts are also used in Vaxelis as an ingredient to "help boost the body's response to the vaccine." Neither ingredient is seen in doses that would be considered toxic, an infectious disease expert told Lead Stories. The Food and Drug Administration documentation contains no evidence that suggests otherwise for this vaccine.
The claim originated in a video shared on Instagram on December 28, 2023 (archived here). In the video, a person said, "Not to mention all the toxic ingredients involved in this vaccine, including formaldehyde and 319 micrograms of aluminum in each dose."
The caption that accompanied the post read:
Not to mention side effects listed in the inserts themselves..
Including hypersensitivity (rash, urticaria, dyspnea, anaphylactic reaction, extensive swelling of injected limb (including swing of adjacent joints), seizures and hypotonic-hyporesponsive episodes.
This is how the post appeared at the time of writing:
(Source: Instagram screenshot taken Wed Jan 2 13:49:00 UTC 2023)
Vaxelis is the first of its kind of vaccine
Vaxelis (archived here) is a hexavalent, or six-in-one, vaccine that protects against diphtheria, tetanus, pertussis, polio, Hib disease (Haemophilus influenzae type b) and hepatitis B. Such combination vaccines put two or more vaccines that could be given individually into a single shot. This convenience is why they are recommended (archived here) over single-dose vaccines.
The Food and Drug Administration (FDA) approved Vaxelis (archived here) in December 2018 for manufacture and sale. In 2021, it became available in the U.S. for use in children between the ages of 6 weeks and 4 years, according to the Children's Hospital of Philadelphia (archived here).
Vaxelis is listed as a recommended vaccine by the American Academy of Pediatrics (archived here) and Centers for Disease Control and Prevention (CDC) (archived here), among other groups.
Dr. William Schaffner (archived here), a professor of medicine in the division of infectious diseases at Vanderbilt University Medical Center, told Lead Stories that the vaccine has been tested against similar products and has been clinically proven as an effective preventative immunization.
Toxicity: All 'a matter of dose'
Health officials and regulators consider certain amounts of each to be safe in vaccines. Although aluminum salts and formaldehyde are listed in the FDA's Vaselix ingredient list (archived here), "toxicity is a matter of dose," Schaffner told Lead Stories in a January 4, 2024, phone interview.
Trace amounts of formaldehyde and aluminum salts are added either to stabilize vaccine materials or remain in small residual concentrations left over from the manufacturing process, Schaffner told Lead Stories.
The CDC lists formaldehyde (archived here) as a "residual inactivating ingredient" that is used to kill viruses or inactivate toxins during the manufacturing process. The agency writes that:
Formaldehyde is diluted during the vaccine manufacturing process, but residual quantities of formaldehyde may be found in some current vaccines. The amount of formaldehyde present in some vaccines is so small compared to the concentration that occurs naturally in the body that it does not pose a safety concern.
Aluminum salt is also classified by the CDC as an ingredient to "help boost the body's response to the vaccine."
"Many vaccines contain these amounts, which have been given in literally billions of doses over decades," Schaffner said. "It's really quite clear that they are not a safety issue in any acute or long-term sense."
In an email to Lead Stories received on January 5, 2024, a spokesperson for Vaxelis' manufacturer, Sanofi, citing a 2013 study published in the peer-reviewed journal Vaccine (archive), wrote that both formaldehyde and aluminum salts have a "long history of safe use." The spokesperson said:
Formaldehyde is removed from the final vaccine product so only very small residual amounts may be present. The amount of formaldehyde present in infant vaccines is much smaller than the concentrations that occur naturally in the human body.
Vaxelis was NOT 'clinically tried against an inert placebo'
The person in the video who is the subject of this fact check stated that Vaxelis was "clinically tried against an inert placebo," but without providing proof.
In fact, since Vaxelis is a first-of-its-kind shot in the U.S., it was not compared with another six-in-one vaccine at the time of its FDA approval because there were no others available on the market.
However, Vaxelis uses the same components as previously approved standard vaccines, rolled into one. And it was tested against similar vaccines.
The Sanofi spokesperson confirmed that "all new vaccines are always tested against the current standard of care vaccines." Testing the vaccine "'against an inert placebo' would be considered unethical as it would place those infants who received a placebo at risk of preventable diseases," he emphasized.
Vaxelis and other routine childhood vaccines, for diseases ranging from tetanus to hepatitis B, were given to trial participants concurrently and compared against the control group, Schaffner said. "There was no trial where the control children received nothing or an 'inert' placebo," he said.
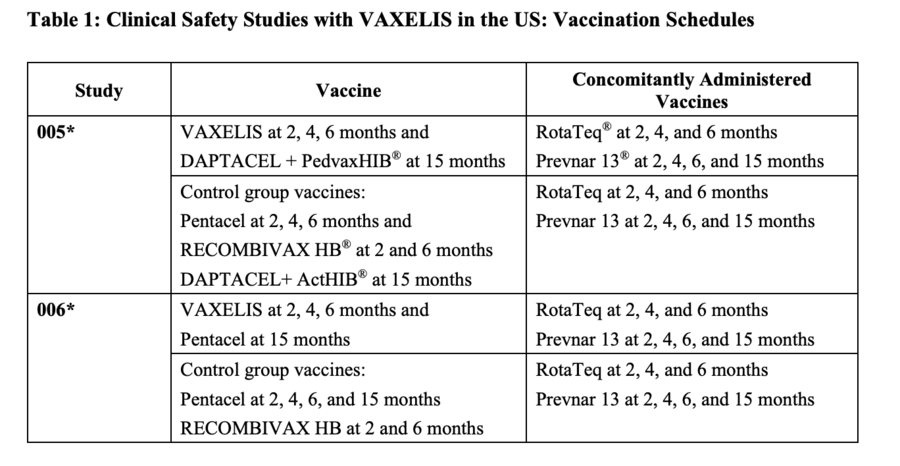
The FDA packaging insert for Vaxelis (archived here) lists six clinical trials. The information for two of those trials, conducted in the U.S. with 3,380 infants, is shown below.
(Source: Vaxelis FDA packaging insert screenshot taken Tues Jan 4 at 23.57.53 UTC 2023)
Six infant deaths were NOT considered related to Vaxelis vaccination
Six deaths were reported among those children who took part in the two U.S. studies, the FDA packaging insert for Vaxelis states. This accounted for 0.2 percent of the total participants.
"Toxic" levels of formaldehyde and aluminum were not cited as the reason for any of these deaths, which the FDA did not consider vaccine-related. The causes of death include Sudden Infant Death Syndrome, asphyxia, unknown cause and the neurological disorder hydrocephalus.
"Across all six clinical studies, there were no deaths assessed as related to VAXELIS," the insert reads.
Other Lead Stories fact checks of claims related to childhood vaccines can be found here.