Did Bill Gates invent an implantable, wireless "birth control microchip"? No, that's not true: Although the Bill and Melinda Gates Foundation has awarded grants to companies working on an implantable contraception device, there is no evidence that Gates personally developed the device. A version of the device was in the preclinical trial stage at the time of the writing of this fact check but it is not available on the market.
The claim appeared in a post (archived here) on Facebook on September 18, 2024. The post contained an image with text that read:
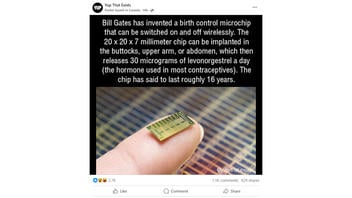
Bill Gates has invented a birth control microchip that can be switched on and off wirelessly. The 20 x 20 x 7 millimeter chip can be implanted in the buttocks, upper arm, or abdomen, which then releases 30 micrograms of levonorgestrel a day (the hormone used in most contraceptives). The microchip has said to last roughly 16 years.
This is what the post looked like on Facebook at the time of writing:
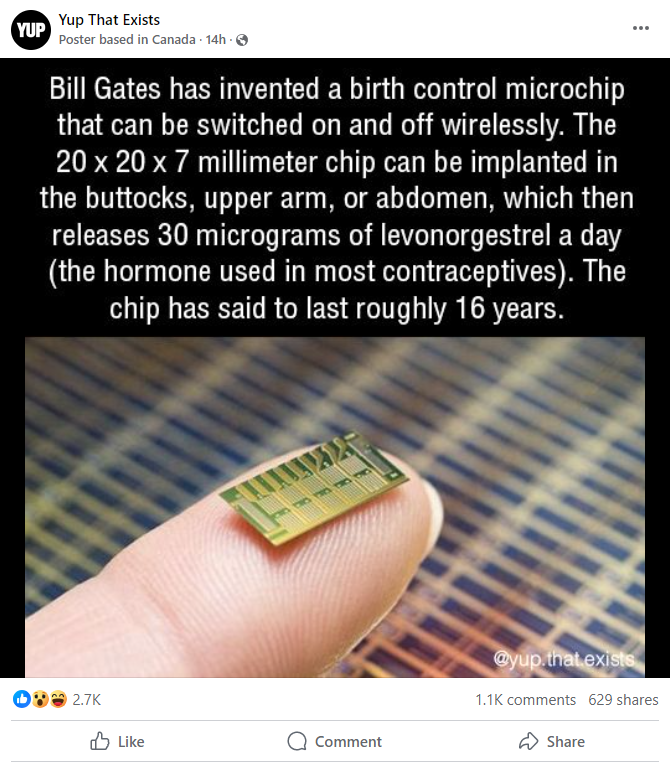
(Source: Facebook screenshot taken on Thu Sept 19 16:02:16 2024 UTC)
Claim incorrectly represents info from articles
An article (archived here) published by the BBC in 2014 describes the device, which is likely where the post making the claim got some of its information and photo. However, the post making the claim misrepresented the article's statement about Gates, which only says that the Microsoft founder "backed," not invented, the idea. The relevant excerpt of the article is included below:
A contraceptive computer chip that can be controlled by remote control has been developed in Massachusetts.
The chip is implanted under a woman's skin, releasing a small dose of levonorgestrel, a hormone.
This will happen every day for 16 years, but can be stopped at any time by using a wireless remote control.
The project has been backed by Bill Gates, and will be submitted for pre-clinical testing in the US next year - and possibly go on sale by 2018.
The device measures 20mm x 20mm x 7mm and will be 'competitively priced', its creators said.
Another article (archived here) published by MIT Technology Review in 2014 contained some of the other information contained in the post making the claim, including the dosage of the levonorgestrel hormone and where the device could be implanted. The article also reported that Gates was involved in the project early on, but not that he invented it:
The idea for the device originated two years ago in a visit by Bill Gates and his colleagues to Robert Langer's MIT lab. Gates and his colleagues asked Langer if it were feasible to create birth control that a woman could turn on and off and use for many years. Langer thought the controlled release microchip technology he invented with colleagues Michael Cima and John Santini in the 1990s and licensed to MicroCHIPS might offer a solution.
History of the 'birth control microchip'
As mentioned in the MIT Technology Review excerpt above, the technology was licensed to an MIT-affiliated startup named MicroCHIPS. A clinical trial (archived here) of the device with osteoporosis patients was conducted under the European Union's oversight (archived here) between 2010 and 2011. Still, the trial did not lead to the widespread release of the device. MicroCHIPS, which was renamed Microchips Biotech, also received grant funding (archived here) from the Gates Foundation in 2012 and 2014 to develop the device.
Microchips Biotech was bought by Daré Bioscience in 2019 (archived here). Daré Bioscience took on the project and called it Dare-LARC1, a "user-controlled, long-acting reversible contraception" (archived here). According to Daré Bioscience's website, "There are currently no FDA-approved contraceptive implants available that allow one to remotely pause and resume dosing," with Dare-LARC1 being in the preclinical trial stage (archived here). The Gates Foundation funded the project in 2021 (archived here).
In an email sent to Lead Stories on September 19, 2024, Robert Langer, the David H. Koch Institute Professor at MIT and former associate of MicroCHIPS, told us that the claim was not accurate.
Lead Stories could not find evidence that the device has been approved by any regulatory body, including the U.S. Food and Drug Administration. We reached out to the Gates Foundation, Daré Bioscience and other individuals associated with Microchips Biotech for comment on the claim. We will update this story with any relevant responses.
Other Lead Stories fact checks
Lead Stories has debunked numerous claims related to both Bill Gates and microchips.