STORY UPDATED: check for updates below.
Did a study published in January 2025 definitively establish a link between Ozempic and blindness, as was implied in a post on X? No, that's not true: The study was a small observational analysis of just nine patients that did not establish that the weight-loss drug caused blindness but instead found an association between the two. In other words, it is not known whether the drug, or others like it, caused the vision loss seen in the patients, or that was caused by other underlying health concerns.
The claim appeared in a post (archived here) on X on February 11, 2025, with a caption that read:
Big Pharma needs to be dismantled.
This is how the post appeared on X at the time of writing:
(Source: X screenshot taken Tue Feb 11 08:53:00 2025 TUC)
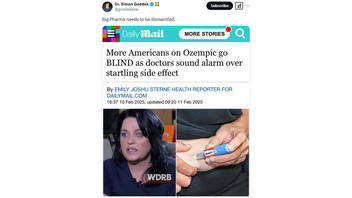
The post included a screenshot of an article published on February 10, 2025, by The Daily Mail originally titled, "More Americans on Ozempic go BLIND as doctors sound alarm over startling side effect."
As of this writing, the headline published by The Daily Mail had been updated to specify that the side effects were only potential, now reading, "More Americans on Ozempic go BLIND as doctors sound alarm over potentially startling side effect."
By sharing the screenshot of The Daily Mail article and the caption, "Big Pharma needs to be dismantled," the post implied that the study concluded that Ozempic caused blindness.
That's false.
The research cited in The Daily Mail article described an observation study. The conclusion section of the study's abstract stated:
In this case series study, it was not possible to determine if there is a causal link between these drugs and the ophthalmic complications reported. In some cases, it is hypothesized that rapid correction of hyperglycemia induced by these drugs, rather than a toxic effect of the drugs, could be associated with the ophthalmic complications reported.
In other words, the researchers did not definitively conclude that the drugs caused the listed ophthalmic complications reported. Rather, it could be that the eye concerns were caused by the rapid correction of high blood sugar levels induced by the drugs.
The study, titled "Ophthalmic Complications Associated With the Antidiabetic Drugs Semaglutide and Tirzepatide" was published in the peer-reviewed journal JAMA Opthalmology on January 30, 2025 (archived here).
Semaglutide, sold under the brand names Ozempic, Rybelsus and Wegovy, is a type of glucagon-like peptide-1 (GLP-1). Tirzepatide is sold under the brand name Mounjaro. Ozempic is a prescription medication used to treat type 2 diabetes and obesity in adults.
Researchers examined a case series describing the reports of patients with related or similar medical histories. It does not necessarily establish a cause-and-effect relationship but describes the background of a medical condition.
Study did not establish a causal link
This series included nine patients, seven described with nonarteritic anterior ischemic optic neuropathy (NAION), which causes sudden vision loss in one eye. Another patient was described with papillitis, inflammation of the optic nerve head, while another patient had paracentral acute middle maculopathy associated with retinal blood flow issues that can cause a blind spot in vision. Each of these patients was using either semaglutide or tirzepatide.
The University of Buffalo published a press release announcing the study's findings on February 7, 2025. It noted that "while reports of patients on semaglutide and tirzepatide with vision problems are still rare, there are concerns, the authors say, because demand for the drugs is skyrocketing."
Kaiser Family Fund published data in May 2024 reporting that roughly 12 percent of U.S. adults had taken a GLP-1 agonist, a class of medications used to treat type 2 diabetes and obesity commonly referred to as "Ozempic."
The study also had limitations that provided important context for its findings. For one, all patients in the study were over the age of 50 -- except one in their 30s and another in their 40s -- and were prescribed the drugs because they had diabetes or obesity, as well as other cardiovascular comorbidities that can cause vision issues. Diabetic patients who aren't on the drugs may also have vision changes due to fluctuations in their blood sugar.
The limitations of the study section read, in part:
This study has some limitations. A retrospective uncontrolled case series is limited by its lack of a control group, potential selection bias, and reliance on historical data, which may lead to incomplete or inaccurate information and reduced ability to establish causality. Because the study was initiated after a single case of NAION associated with semaglutide, there is a risk of confirmation bias. A future study, with a broader scope to explore other, potential ocular adverse effects from GLP1-RAs with a hypothesis-driven approach, could provide stronger and more comprehensive evidence.
The full text of the study can be read here (archived here).
Further research is required to establish possible causal relationships
Dr. Norah Lincoff, a neuro-ophthalmologist at the University of Buffalo, said in the study press release that the study authors are "joining with the American Academy of Ophthalmology in recommending a post-marketing survey to more accurately assess the number of patients receiving these drugs who experience an adverse ocular reaction."
Essentially, more research is needed to establish a connection between GLP-1 drugs and potential associated vision issues.
Lead Stories contacted lead study authors Lincoff, Dr. Bradley Katz and Dr. Michael Lee for comment. We will update this article accordingly.
Preliminary research shows association between GLP-1, vision concerns
Some medical experts have found an association between GLP-1 agonists and vision issues associated with diabetes, but whether the drug or symptoms of the disease caused these impairments require further research.
For example, research published in September 2024 "found that the use of GLP-1 agonists may be associated with increased risk of vision-threatening complications."
Another study announced in July 2024 suggested that "patients taking semaglutide ... may be at higher risk of developing an eye condition that can cause blindness."
Read more
Other Lead Stories fact checks involving health topics can be read here.
Updates:
-
2025-02-12T18:21:23Z 2025-02-12T18:21:23Z Adds link to full study preview.