STORY UPDATED: check for updates below.
The British Medical Journal published an open letter on December 17, 2021 (archived here) taking issue with a Lead Stories fact check. Our response is below (UPDATE: also see "Context Matters: Why Lead Stories Fact Checked The BMJ"):
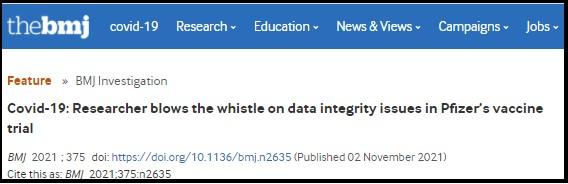
It is ironic to read that BMJ.com objects to the headline on Lead Stories' fact check of a BMJ.com article when the original BMJ piece carries a scare headline that oversells the whistleblower and overstates the jeopardy. Their November 2, 2021, headline "Covid-19: Researcher blows the whistle on data integrity issues in Pfizer's vaccine trial" is the reason BMJ.com's article has appeared in hundreds of Facebook posts and tweets, many by anti-vaccine activists using it as "proof" the entire clinical trial was fraudulent and the vaccine unsafe.
Likely unknown to the BMJ was the fact that the publication of their article happened to coincide with a hugely viral story making the rounds in anti-vaccine circles falsely claiming the CEO of Pfizer was arrested for fraud.
The combination of these two factors lead to enormous engagement by Facebook users on the BMJ article according to CrowdTangle data:
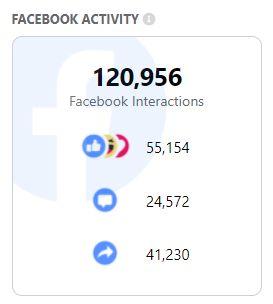
(Source: CrowdTangle screenshot taken Sat, 18 Dec 2021 10:30:33 UTC)
That heavy traffic is why BMJ's claims were called to our attention. Part of our mission is to fight what the World Health Organization says is an "infodemic" of misleading claims about COVID-19. Through Facebook's Third-Party Fact-Checking Program, our work is used to slow the spread of outright falsehoods, or to warn readers to think about crucial context that's missing in other posts. In this case, Facebook users seeing BMJ.com's article are merely warned of "Missing Context", the lightest measure Facebook applies, with no restrictions in traffic, visibility or advertising revenue.
The "Missing Context" label, according to Meta's Rating Options for Fact-Checkers, is meant for:
Content that may mislead without additional context.
Note that there are separate rating options available for "False" "Partly False" and "Altered" content and that we did not select any of these options for rating the BMJ article.
The "Missing Context" label applies to content that (while true or real) might still be misleading because crucial information is missing. Given the enormous engagement the article received and the kinds of reactions it elicited that certainly seems to have been the case here.
(Source: Archive.org screenshot taken Sat. Dec. 18 23:46:50 2021 UTC)
The BMJ.com headline, shown above, fails to make two important distinctions:
- The allegation concerns just three of the 153 sites at which the vaccine was tested on 44,000 participants. It would have been less misleading to say "data integrity issues at 3 of 153 Pfizer trial sites."
- BMJ.com's whistleblower is not a lab-coated scientist titrating doses or checking patient symptoms. By her own account, Brook Jackson holds a 30-hour certification in auditing techniques focused on proper electronic medical records and data capture as well as lab procedures.
The BMJ.com article eventually gets around to saying she worked at the lab for just two weeks. But BMJ's open letter fails to mention important context: The Brook Jackson Twitter account agreed with leading COVID misinformation-spreader Robert F. Kennedy Jr.'s criticism of the "Sesame Street" episode in which Big Bird encourages kids to get a COVID-19 vaccine. "Shocking, actually." she wrote in a November 9, 2021, response to a Kennedy tweet blasting Sesame Street (archived here). Elsewhere on Twitter, the Brook Jackson account wrote to a vaccine-hesitant person that vaccination makes sense if a person is in a high-risk category. When the U.S. 5th Circuit Court of Appeals ruled against a federal employee vaccine mandate, she tweeted "HUGE!" and not with a frowny emoji.
Lead Stories talked to Jackson, looked at available documents (after BMJ refused to permit us to see their basis for the story and did not make the documents available on a transparency site). Unlike BMJ.com, Lead Stories then tested Jackson's assertions with Pfizer, with the lab contractor in question and with the FDA and then published their responses. It's not at all clear yet whether there are data integrity issues if you ask the other stakeholders, and that's the crucial missing context. We also talked to experienced medical
By talking to Ventavia, we contributed context BMJ.com missed: Ventavia said the whistleblower had not worked on the Pfizer trial, but Lead Stories set that straight by embedding in its story a copy of a letter, provided by Jackson in which she was expressly welcomed to the Pfizer trial team. That's what we mean by context.
The BMJ has thus far failed to document what is "inaccurate" in the Lead Stories fact check, but again oversells by using that and other name-calling to vent frustration at our documentation of obvious missing context:
- BMJ.com objects to our use of the term "news blog." Without a hard copy in hand, we can only responsibly work from the BMJ.com post. It is one item in a regularly updated feed of articles with the newest items at the top: a news blog, subject to the kind of after-the-fact updating not possible in a printed journal.
- As we explained to BMJ.com in a lengthy exchange of emails, our headline describes what we found: Even if true, the problems likely do NOT disqualify the findings of the whole Pfizer trial and they were NOT provably ignored. Both Pfizer and the U.S. Food and Drug administration told us they have been aware of and looking into the questions raised by the auditor involved in three of 153 sites of a 44,000-person clinical trial. We await results.
- We agree the 14-character caption over our thumbnail image is not detailed. But it summarizes what we found: Flaws flagged by the auditor have been and are being reviewed. Hence. "Flaws Reviewed."
- "Missing Context" is the most accurate rating available to us in the Facebook Fact-Checking rubric: The concerns Jackson raised affect three of 153 sites, while BMJ.com's headline makes no effort to convey how small the subset is; at no point in the article are Pfizer, Ventavia and the FDA granted the opportunity to respond to accusations of mis-, mal- and non-feasance; the whistleblower's public statements about vaccines give vital context to her actions; and while Jackson was hired for her auditing expertise, her publicly expressed views of COVID vaccines, public health efforts and of vaccination of children are at least noteworthy. BMJ prides itself on accompanying each article with a statement of conflicts of interest. Perhaps its high-level editing could have noted their key source's advocacy.
For the Lead Stories Editorial Team:
Dean Miller (Managing Editor), Alan Duke (Co-founder and Editor-in-Chief), Maarten Schenk (Co-founder and CTO/COO)
P.S.: The open letter also calls out Meta for blocking Instagram mentions of Cochrane, based on content that allegedly violates "Community Guidelines".
🤔 Um, @instagram you got this one wrong! @cochranecollab and @CochraneLibrary continue to be there for those looking to use high-quality information to make #health decisions. Learn more: https://t.co/8q0IowNp9u And search our evidence: https://t.co/3AdemrcQSL #infodemic pic.twitter.com/m6NUItZ3tu
-- Cochrane (@cochranecollab) November 10, 2021
We would like to point out that Lead Stories is not involved in enforcing said Community Guidelines and that we agree this content probably should not be blocked. We actually alerted our contact at Meta about this issue on November 10, 2021 and hope this can be resolved soon.
Update: it appears the issue with Cochrane was already resolved on November 23, 2021, weeks before the BMJ open letter (and weeks *after* Lead Stories reported the issue to Meta).
Cochrane's 'shadow ban' on Instagram has finally been removed after several weeks! But how do you encourage a safe and evidence-based social media platform? We would love to hear your thoughts on this. https://t.co/EbWSJOVtSy
-- Cochrane (@cochranecollab) November 23, 2021
(Editors' Note: Meta is a client of Lead Stories, which is a third-party fact checker for the social media platform. On our About page, you will find the following information:
Since February 2019 we are actively part of Facebook's partnership with third party fact checkers. Under the terms of this partnership we get access to listings of content that has been flagged as potentially false by Meta's systems or its users and we can decide independently if we want to fact check it or not. In addition to this we can enter our fact checks into a tool provided by Meta and Meta then uses our data to help slow down the spread of false information on its platforms. Meta pays us to perform this service for them but they have no say or influence over what we fact check or what our conclusions are, nor do they want to.)
Updates:
-
2022-01-28T11:43:30Z 2022-01-28T11:43:30Z Added link to "Context Matters" piece. -
2022-01-04T19:11:46Z 2022-01-04T19:11:46Z Added an update on the issue with Cochrane.