STORY UPDATED: check for updates below.
Update: In response to this fact check the BMJ published an open letter to Mark Zuckerberg. Our response is here, we stand by our reporting. We also published an article titled "Context Matters: Why Lead Stories Fact Checked The BMJ" to further clarify the reasons for the fact check after the BMJ published another article claiming this was an case where "fact checking goes wrong". Obviously we don't agree.
Did the British Medical Association's news blog reveal flaws that disqualify the results of a contractor's field testing of Pfizer's COVID-19 vaccine, and were the problems ignored by the Food & Drug Administration and by Pfizer? No, that's not true: Pfizer and the FDA were made aware of the allegations about the contractor in 2020. Medical experts say the claims aren't serious enough to discredit data from the clinical trials, which is also what Pfizer and the FDA say they concluded. The FDA says its position is unchanged: The benefits of the Pfizer vaccine far outweigh rare side effects and the clinical trial data are solid.
The claims were made in a November 2, 2021, article on the BMJ blog titled "Covid-19: Researcher blows the whistle on data integrity issues in Pfizer's vaccine trial" (archived here), which opened:
... for researchers who were testing Pfizer's vaccine at several sites in Texas during that autumn, speed may have come at the cost of data integrity and patient safety.
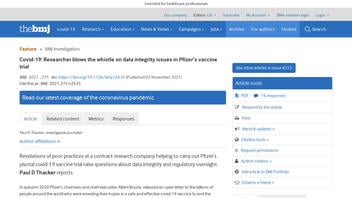
Users on social media only saw this title, description and thumbnail:
Covid-19: Researcher blows the whistle on data integrity issues in Pfizer's vaccine trial
Revelations of poor practices at a contract research company helping to carry out Pfizer's pivotal covid-19 vaccine trial raise questions about data integrity and regulatory oversight. Paul D Thacker reports In autumn 2020 Pfizer's chairman and chief executive, Albert Bourla, released an open letter to the billions of people around the world who were investing their hopes in a safe and effective covid-19 vaccine to end the pandemic. "As I've said before, we are operating at the speed of science," Bourla wrote, explaining to the public when they could expect a Pfizer vaccine to be authorised in the United States.1 But, for researchers who were testing Pfizer's vaccine at several sites in Texas during that autumn, speed may have come at the cost of data integrity and patient safety. A regional director who was employed at the research organisation Ventavia Research Group has told The BMJ that the company falsified data, unblinded patients, employed inadequately trained vaccinators, and was slow to follow up on adverse events reported in Pfizer's pivotal phase III trial. Staff who conducted quality control checks were overwhelmed by the volume of problems they were finding. After repeatedly notifying Ventavia of these problems, the regional director, Brook Jackson, emailed a complaint to the US Food and Drug Administration (FDA). Ventavia fired her later the same day. Jackson has provided The BMJ with dozens of internal company documents, photos, audio recordings, and emails. On its website Ventavia calls itself the largest privately owned clinical research company in Texas and lists many awards it has won for its contract work.2 But Jackson has told The BMJ that, during the two weeks she was employed at Ventavia in September 2020, she repeatedly informed her superiors of poor laboratory management, patient safety concerns, and data integrity issues. Jackson was ...
The BMJ reported patient safety and data integrity were likely compromised by the practices of Ventavia Research Group, a contractor that oversaw three of the 153 sites where Pfizer trials on 46,000 patients were conducted. BMJ relied on copies of reports filed by a two-week employee of Ventavia.
Medical experts disagree with claims that this contretemps calls into question the results of the Pfizer clinical trial.
Professor Douglas Drevets, M.D., of the University of Oklahoma College of Medicine, wrote in a November 10, 2021, email to Lead Stories that even if the claims are true, there is abundant proof the Pfizer vaccine works and is safe. Drevets, who heads the infectious diseases department at University of Oklahoma Health Sciences Center, said the millions of doses administered more than prove this:
... there have been so many other studies of the Pfizer COVID-19 vaccine since the Phase III trial that people can be confident in its efficacy and safety profile. That said, Pfizer might be wise to re-run their analysis excluding all Ventavia subjects and show if that does/does not change the results. Such an analysis would give added confidence in the Phase III results.
In an article in MedPage, vaccine experts said the claims are not serious enough to jeopardize Pfizer's data.
The BMJ article says a former Ventavia worker named Brook Jackson told The BMJ that Ventavia falsified data, "unblinded" patients and employed inadequately trained vaccinators. Under National Institutes of Health standards, a "double-blind" clinical trial is one in which neither the patient nor the person giving the medicine knows if the patient is getting a placebo or the actual drug.
The BMJ reported:
Jackson has provided The BMJ with dozens of internal company documents, photos, audio recordings, and emails ... Jackson has told The BMJ that, during the two weeks she was employed at Ventavia in September 2020, she repeatedly informed her superiors of poor laboratory management, patient safety concerns, and data integrity issues.
The FDA said, without explicitly saying it, that the allegations don't change the agency's assessment of the vaccine's safety. FDA spokesperson Alison Hunt wrote in a November 10, 2021, email to Lead Stories that the FDA still declares that the benefits outweigh risks that come with Pfizer's vaccine. Hunt wrote:
Although the agency cannot comment further at this time in this ongoing matter, FDA has full confidence in the data that were used to support the Pfizer-BioNTech COVID-19 Vaccine authorization and the Comirnaty approval.
A spokesperson for Texas-based Ventavia Research Group wrote in a November 10, 2021, email to Lead Stories that BMJ did not seek comment in advance of the report. If it had, it would have been told the employee's report was investigated but found wanting, the spokesperson said. Lauren Foreman, director of business development & communications, wrote:
The accuser was employed for approximately two weeks in September 2020, and no part of her job responsibilities concerned the clinical trials at issue. These same accusations were made a year ago, at which time Ventavia notified the appropriate parties. The allegations were investigated and determined to be unsubstantiated. Ventavia takes research compliance, data integrity, and participant safety very seriously, and we stand behind our work supporting the development of life-saving vaccines.
Pfizer said it has reviewed the claims and found them to be unproven. In a November 10, 2021, email to Lead Stories, Pfizer senior manager for science media relations, Kit Longley, detailed Pfizer's response to the claims:
Pfizer received communication from an anonymous complainant in September 2020 relating to a single clinical investigator site in Texas, USA. We conducted a thorough investigation into the issues raised in accordance with Pfizer's quality management process related to clinical research. Actions were taken to correct and remediate where necessary. Pfizer's investigation did not identify any issues or concerns that would invalidate the data or jeopardize the integrity of the study. The company proactively notified the US Food and Drug Administration of the matter and informed the Institutional Review Board for the study.
The Pfizer-BioNTech BNT162b vaccine was subsequently approved by the FDA, EMA and other regulatory authorities based on the robust data submitted from the clinical program. The vaccine has been given to hundreds of millions of people worldwide following approval.
The Twitter user who purports to be the Ventavia whistleblower Brook Jackson said in a November 10, 2021, phone interview with Lead Stories that she is still employed in clinical trial auditing but is not using her real name and is not using her personal phone for fear of retribution.
She said she holds a certificate in clinical trial auditing from Barnett International, which offers a 30-hour course. She said she is fully vaccinated and is not an anti-vaccine activist. "My story is not about whether the vaccine is efficacious. I'm talking about data integrity," she said from a phone number listed to a Dallas suburb.
Responding to comments and inquiries on Twitter, Jackson wrote in a November 9, 2021, tweet: "The organizational failures listed in the BMJ, although not exhaustive, led to violations that were repeated and deliberate, and the reliability and integrity of the data have been comprised."
Her Twitter account, which was created in September 2021, includes recent posts about the BMJ report as well as others that support some elements of vaccine resistance.
Here is the CBER report I filed on 25Sep2020. pic.twitter.com/VtqDLWTCo9
-- Brook Jackson (@IamBrookJackson) November 6, 2021
Clicking on the document, it appears to be an internal Ventavia email welcoming a Brook Jackson and adding her to the team working on the Pfizer trial. This is notable because Ventavia has said she was not part of that team.
Want something more? pic.twitter.com/KmSpn2W5ui
-- Brook Jackson (@IamBrookJackson) November 9, 2021
Ventavia managed 3 of 153 sites at which the trial was carried out. A Pfizer spokesman has promised to provide to Lead Stories an update on the number of trial participants Ventavia enrolled of the 46,000 overall.
On Twitter, Jackson does not express unreserved support for COVID vaccines.
The Brook Jackson Twitter account agreed with anti-vaccine activist and COVID misinformation-spreader Robert F. Kennedy, Jr.'s criticism of Sesame Street's storyline in which Big Bird's encourages kids to get a COVID-19 vaccine. "Shocking, actually." she wrote in a November 9, 2021 response to a Kennedy tweet blasting Sesame Street.
Elsewhere on Twitter, the Brook Jackson account wrote that vaccination makes sense if a person is in a high-risk category and called a 5th Circuit Court of Appeals ruling against the Biden Administration's vaccine mandates "HUGE!"
Updates:
-
2022-01-28T11:46:14Z 2022-01-28T11:46:14Z Added link to "Context Matters" piece. -
2021-12-18T17:09:52Z 2021-12-18T17:09:52Z Added link to BMJ's open letter and our response.