Does European Medicines Agency data show more than 1.1 million adverse drug reactions and more than 30,000 deaths by COVID-19 vaccinations? Yes, that's true: Those numbers are found in EudraVigilance, the European Union's database of suspected adverse drug reaction reports. But the numbers don't tell the full story as the meaning of EudraVigilance reports is unclear because the numbers are unverified, in a system open for anyone to file a report.
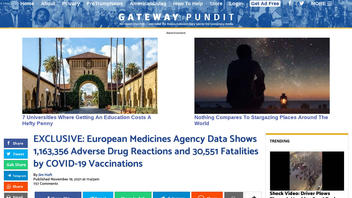
The claim appeared in an article published on the Gateway Pundit website (archived here) on November 19, 2021, titled "EXCLUSIVE: European Medicines Agency Data Shows 1,163,356 Adverse Drug Reactions and 30,551 Fatalities by COVID-19 Vaccinations." It opens:
The official European Union database of suspected drug reaction website is now reporting 30,551 fatalities and 1,163,356 adverse drug reactions from COVID vaccines Pfizer, Moderna, Johnson & Johnson, and AztraZeneca through November 13, 2021 based on the data submitted to its system.
According to European Medicines Agency, an official website of the European Union, the data of adverse reaction from COVID-19 vaccines were posted in ADRreports.eu portal that 'allows users to view the total number of individual suspected side effect reports (also known as Individual Case Safety Reports, or ICSRs).'
This is what the article looked like on the Gateway Pundit website on November 22, 2021:
(Source: Gateway Pundit website screenshot taken on Tue Nov 22 17:51:44 2021 UTC)
These numbers are from the EudraVigilance database run by the European Medicines Agency, an official website of the European Union. While Lead Stories couldn't precisely match the figures, we came up with very similar numbers on November 22, 2021. The numbers cited in the article were through November 13, 2021, according to the Gateway Pundit.
Those reports of adverse drug reactions can be made by anyone with a computer and internet access. It's not limited to health care professionals. The website's disclaimer includes, "The information on this website does not reflect any confirmation of a potential link between the medicine and the observed effect(s)."
EudraVigilance explains how to report a side effect and includes a two-page PDF on how to file for non-health care professions.
A Lead Stories analysis of EudraVigilance data through November 20, 2021, showed this breakdown of the 1,181,121 total adverse cases reported for each manufacturer of COVID-19 vaccines:
Of all the cases filed with EudraVigilance, 64% of the reports (756,309) were from "Non Healthcare Professionals." The remaining 36% (424,812) came from "Healthcare Professionals."
The EudraVigilance database serves a similar function as the Vaccine Adverse Event Reporting System (VAERS) in the United States, which is run by the Centers for Disease Control and Prevention and the Food and Drug Administration.
Lead Stories has debunked several claims about vaccine deaths that misuse VAERS.
Anyone with internet access can add a report to the VAERS list of reports. The public access link to it expressly warns against unwarranted conclusions based on VAERS material because the list only provides a tally of unverified notes about any health event people experience after they are vaccinated.
The list itself cannot be used to prove or quantify, since all it shows is a chronological correlation, not the causal link that would be more difficult to establish. It's the equivalent of a police precinct's running "blotter" of reports that may serve as a starting point for police work, not an endpoint.