Does everyone who got a COVID-19 vaccine need to be tested for HIV? No, that's not true: A COVID vaccine that produced false-positive results for the virus that causes AIDS was used during clinical trials in Australia but later discontinued because of the "risk to vaccine confidence" those test results generated. The vaccine used harmless HIV fragments that produced the false positives.
The claim appeared in a post and video (archived here) on Facebook on February 15, 2022. It opened:
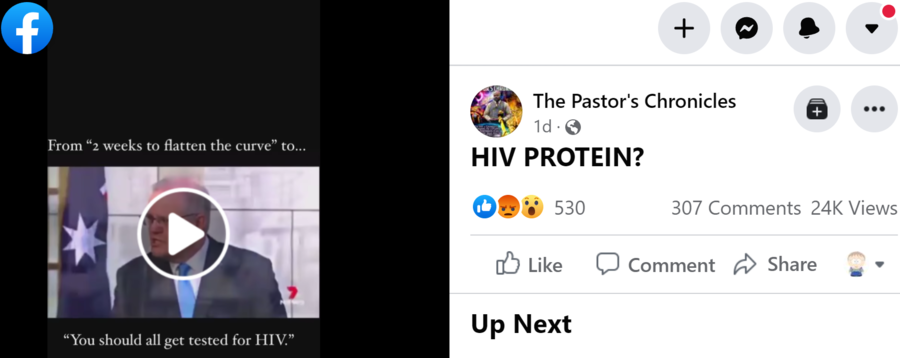
HIV PROTEIN?
From "2 weeks to flatten the curve" to... "You should all get tested for HIV."
This is what the post looked like on February 16, 2022:
(Source: Facebook screenshot taken on Wed Feb 16 21:41:05 2022 UTC)
The video shared in the Facebook post is old and edited. The original was produced by Australia's Seven News in December 2020. The country had just pulled the plug on the University of Queensland/CSL vaccine, which was under clinical trial and producing false positive HIV test results. The reason? The vaccine contained harmless HIV fragments. The clinical trials looked good. The vaccine was effective, but the PR wasn't working out, Minister for Health Greg Hunt said on December 11, 2020:
It's a powerful and important breakthrough as a platform, and we thank our scientists for that. But the issue, which by mutual agreement with CSL led to the decision not to proceed to Stage 3 trials and, therefore, not to move to a purchase, is that there was the risk of false-positive HIV results. They are false. It comes from the protein that was used. And as a result of that, the scientific advice is that the risk to vaccine confidence was the principal issue here, and we made the decision unanimously as a National Security Committee, the scientific advice was unanimous, the agreement with CSL not to proceed was mutual. And this is the scientific process working. It's the planning process working. It's an honest explanation of some of the challenges we've had. But, at the end of the day, 31 million new vaccines purchased for Australia, and the potential for a slightly earlier completion of the rollout with the commencement process still on track for March, subject to the approvals and the news on our vaccine candidates is strong.