Did Nestlé Purina PetCare receive a warning letter from the Food and Drug Administration (FDA) regarding company practices in both 2015 and 2021? No, that's not true: While the FDA did send a warning letter to a Purina plant manager in 2015 about one of the company's canning facilities, the company did not get a warning letter from the FDA in 2021. However, it did issue a voluntary recall of one of its products in 2021.
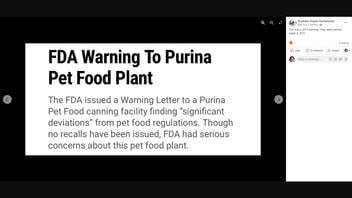
The claim appeared in a Facebook post (archived here) on April 9, 2022. It had a screenshot of a 2015 article titled "FDA Warning To Purina Pet Food Plant." The caption of the post reads:
This was a 2015 warning. They were warned again in 2021.
This is what the post looked like on Facebook at the time of writing:
(Source: Facebook screenshot taken on Wed Apr 13 17:40 2022 UTC)
The FDA sent a warning letter to a Purina plant manager regarding concerns about a canning facility in 2015. The letter cited several regulation violations noted by FDA inspectors at the Pennsylvania facility. The warning was closed out in a separate letter dated December 15, 2015.
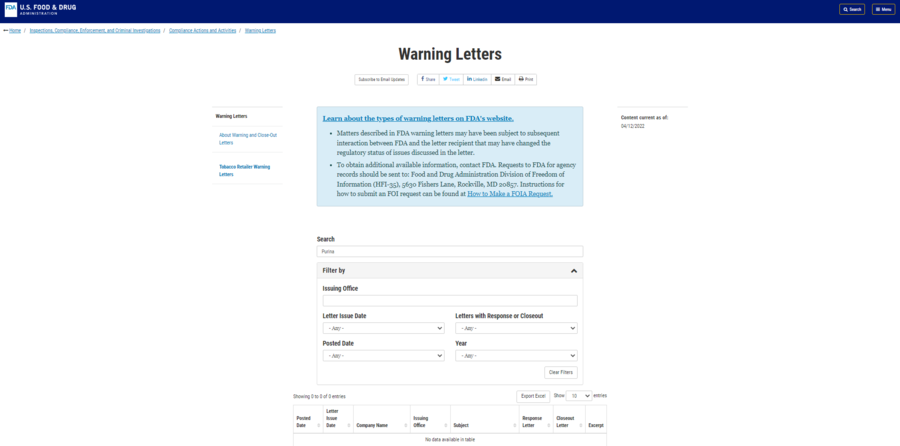
Lead Stories checked the FDA warning letter database to see if a warning letter was sent to Purina in 2021. At the time of writing, the database contained warning letters sent from 2017 to 2022. A search of "Purina" generated no results, as shown in the screenshot below:
(Source: FDA screenshot taken on Wed Apr 13 14:14:37 2022 UTC)
In 2021, however, the company did issue a voluntary, limited recall of its Purina Pro Plan Complete Essentials Tuna Entrée In Sauce Wet Cat Food in 3-ounce cans. The recall was announced on July 14, 2021.