Did the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) find a link between the Pfizer bivalent COVID-19 vaccine and strokes in the elderly? No, that's not true: The U.S. public health agencies did detect a possible safety signal for people 65 and older with the Pfizer shot, but the CDC said, "data currently suggests that it is very unlikely that the signal ... represents a true clinical risk."
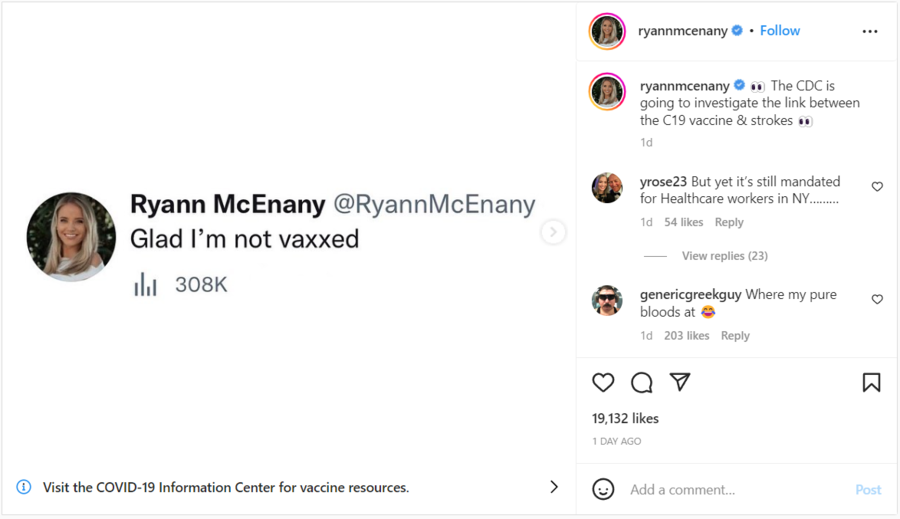
The claim appeared in a post on Instagram on January 14, 2023, under the title "Glad I'm not vaxxed." The caption read:
👀 The CDC is going to investigate the link between the C19 vaccine & strokes 👀
This is what the post looked like on Instagram at the time of writing:
(Source: Instagram screenshot taken on Mon Jan 16 16:40:24 2023 UTC)
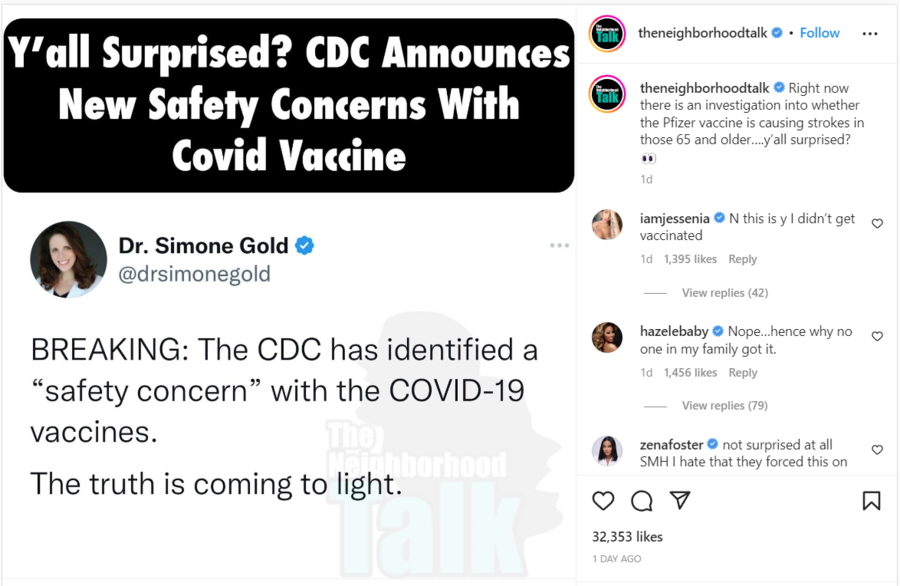
Social media posts surrounding the claim were circulating widely in mid-January 2023. This one was published on Instagram on January 14, 2023:
(Source: Instagram screenshot taken on Mon Jan 16 20:02:09 2023 UTC)
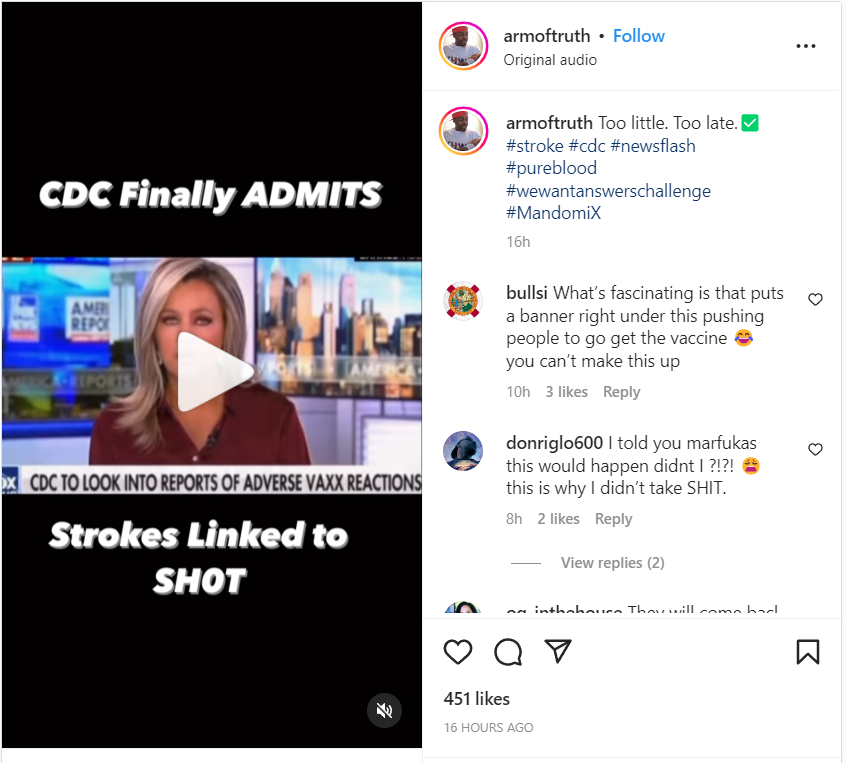
And this one was published on Instagram on January 16, 2023:
(Source: Instagram screenshot taken on Mon Jan 16 21:16:22 2023 UTC)
These Instagram posts refer to a January 13, 2023, release from the CDC and the FDA titled "CDC & FDA Identify Preliminary COVID-19 Vaccine Safety Signal for Persons Aged 65 Years and Older." The government agencies use an early surveillance system to find potential safety concerns that could be flagged early for further investigation. It detected possible "safety signals" for the Pfizer bivalent COVID-19 vaccine, the CDC and the FDA said:
Following the availability and use of the updated (bivalent) COVID-19 vaccines, CDC's Vaccine Safety Datalink (VSD), a near real-time surveillance system, met the statistical criteria to prompt additional investigation into whether there was a safety concern for ischemic stroke in people ages 65 and older who received the Pfizer-BioNTech COVID-19 Vaccine, Bivalent. Rapid-response investigation of the signal in the VSD raised a question of whether people 65 and older who have received the Pfizer-BioNTech COVID-19 Vaccine, Bivalent were more likely to have an ischemic stroke in the 21 days following vaccination compared with days 22-42 following vaccination.
No preliminary signal was identified with the Moderna bivalent COVID-19 vaccine, the release said.
All signals, like the one identified for the Pfizer bivalent vaccine, require further investigation and confirmation from formal epidemiologic studies to determine if there's a causal relationship between the vaccine and the incidence of strokes. As of January 13, 2023, the CDC and the FDA said no other safety systems have shown a similar signal and no other analyses have validated the initial signal:
- A large study of updated (bivalent) vaccines (from Pfizer-BioNTech and Moderna) using the Centers for Medicare and Medicaid Services database revealed no increased risk of ischemic stroke
- A preliminary study using the Veterans Affairs database did not indicate an increased risk of ischemic stroke following an updated (bivalent) vaccine
- The Vaccine Adverse Event Reporting System (VAERS) managed by CDC and FDA has not seen an increase in reporting of ischemic strokes following the updated (bivalent) vaccine
- Pfizer-BioNTech's global safety database has not indicated a signal for ischemic stroke with the updated (bivalent) vaccine
- Other countries have not observed an increased risk for ischemic stroke with updated (bivalent) vaccines
Through the investigation, the agencies determined "the totality of the data currently suggests that it is very unlikely that the signal in VSD represents a true clinical risk."
A January 12, 2023, article by Lead Stories had similar findings: "Fact Check: FDA Study Does NOT Prove Pfizer COVID Vaccine Increases Risk Of Lung Blood Clots 50% In Elderly."
Contacted for a response to the claim, Pfizer media relations provided this statement to Lead Stories on January 16, 2023:
Pfizer and BioNTech have been made aware of limited reports of ischemic stroke that have been observed in the CDC Vaccine Safety DataLink (VSD) database in people 65 and older following vaccination with the Omicron BA.4/BA.5-adapted bivalent COVID-19 Vaccine by Pfizer and BioNTech.
Neither Pfizer and BioNTech nor the CDC or the U.S. Food and Drug Administration (FDA) have observed similar findings across numerous other monitoring systems in the U.S. and globally and there is no evidence to conclude that ischemic stroke is associated with the use of the companies' COVID-19 vaccines. Compared to published incidence rates of ischemic stroke in this older population, the companies to date have observed a lower number of reported ischemic strokes following the vaccination with the Omicron BA.4/BA.5-adapted bivalent vaccine.
The CDC continues to recommend vaccination with the Pfizer-BioNTech Omicron BA.4/BA.5-adapted bivalent COVID-19 vaccine for all authorized ages and indications. The original vaccine is recommended for everyone 6 months old and older.
Additional Lead Stories fact checks of claims about COVID-19 vaccination can be found here.