Did a Pfizer representative "admit" the company erred when its COVID-19 vaccine was "never tested on preventing transmission" of the virus during clinical trials? No, that's not true. Vaccine clinical trials for drug approval are not meant to test that. Clinical trials are intended to check the safety and efficacy of new drugs and vaccines before they are approved for widespread use. Testing for the prevention of disease transmission is not typically part of initial trials, according to vaccine experts. In this case, the vaccine's ability to prevent transmission was assessed later in the roll-out of the vaccine, which was developed in response to a worldwide pandemic.
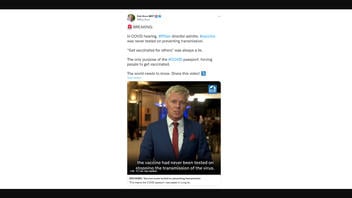
The claim originated in a video posted to Twitter on October 11, 2022, by Dutch politician Rob Roos. The post (archived here) read:
🚨 BREAKING:
In COVID hearing, #Pfizer director admits: #vaccine was never tested on preventing transmission.
"Get vaccinated for others" was always a lie.
The only purpose of the #COVID passport: forcing people to get vaccinated.
The world needs to know. Share this video! ⤵️
Here is a screenshot of the tweet at the time of writing:
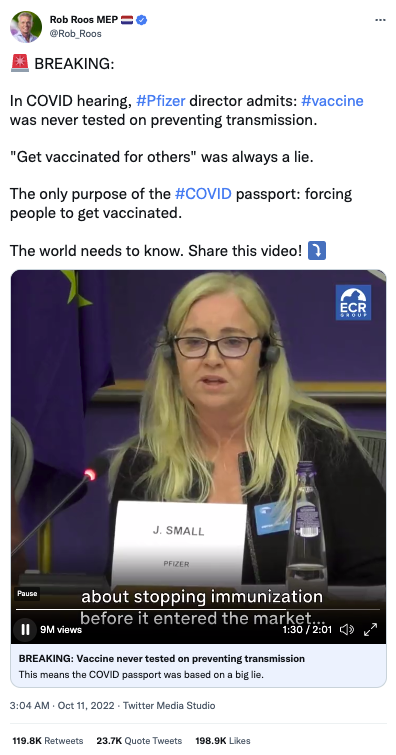 (Source: Twitter screenshot taken on Weds Oct 12 20:52:33 2022 UTC)
(Source: Twitter screenshot taken on Weds Oct 12 20:52:33 2022 UTC)
The claim relies on an excerpt from a video of a meeting of the European Parliament's special committee on the COVID-19 pandemic, looking at lessons learned and recommendations for the future. Held in Brussels on October 10, 2022, the meeting was titled, "COVID-19: debate with the pharmaceutical industry and was described as follows:
Representatives from Pfizer and Curevac share their views on past and present manufacturing, distribution and equitable access of the COVID-19 vaccines and therapeutics. The debate focuses on the activities undertaken to develop vaccines for new variants, the authorisation process, and transparency of contracts. Extracts from the exchange of views with Janine Small, President of International Developed Markets, Pfizer and Dr Franz-Werner Haas, Chief Executive Officer, Curevac.
Lead Stories contacted the European Parliament for the full video and was referred to this link (archived here). Our newsroom then transcribed the exchange between European Parliament member Roos and Janine Small, Pfizer president of international developed markets, which began at the 15:21:24 mark:
Roos: [15:22:56] So there are no misunderstandings, was the Pfizer Covid vaccine tested on stopping the transmission of the virus before it entered the market? If not, please say it clearly. If yes, are you willing to share the data with this committee? And I really want straight answer. Yes or no, and I'm looking forward to it. Thank you very much.
Small: [15:31:47] Regarding the question around did we know about stopping immunization [transmission] before it entered the market. No. These, uhm, you know, we had to really move at the speed of science to really understand what is taking place in the market and from that point of view we had to do everything at risk. I think Dr. [Albert] Bourla - even though he's not here - would turn around and say to you himself, "if not us then who?"
Clinical trials do not test transmissibility
While Pfizer and Moderna vaccines were shown to protect against illness and severe disease, the Association of American Medical Colleges notes vaccine clinical trials are not "designed to test whether any of the trial participants contracted COVID-19 but showed no symptoms."
In short, the trials that tested the safety and efficacy of the vaccine were not designed to test transmission in part because the trial size and duration would have needed to be larger and longer and the goal was to prevent deaths.
It is not true that the vaccines' ability to prevent transmission was never studied.
A short 2021 report in the journal Inflammopharmacology, summarized early findings about the effectiveness of various COVID-19 drugs in preventing the spread of the virus and found it likely did prevent transmission, theorizing:
The molecular hypothesis underlying these results, suggest that in the vaccinated subject and COVID-19 positive may be present the virus, structurally intact, but immediately covered with antibodies of the subject, which make the virus unable to infect other people.
The New England Journal of Medicine looked at the question in a January 2022 article, finding vaccination against the alpha variant reduced transmission better than vaccination against the delta variant.
Vaccines require three phases of clinical trial testing
The Centers for Disease Control and Prevention (CDC) notes that new vaccines are generally developed in three phases, none of which are designed to test transmission.
-
Phase 1: Initial assessment involving a small group (20 to 100) of healthy volunteers who have not been exposed to the virus. This is simply to determine if there are adverse reactions to a vaccine being tested.
-
Phase 2: A larger study containing hundreds of volunteers with varying health backgrounds to test the immune response to the vaccine. Additional safety information on short-term effects, risks, and effectiveness is also collected.
-
Phase 3: The vaccine is administered to thousands of people to determine whether it protects those who are vaccinated from becoming sick compared to those who were not vaccinated. This determines safety profiles and how well the vaccine protects the immunized, as well as provides additional information on less common side effects.
From the COVID19 Vaccine Tracker at McGill University, a collaborative project by public health and infectious disease experts:
Most phase 3 clinical trials report the vaccine efficacy (VE) for symptomatic disease as the primary outcome. That tells us how well the vaccine prevents us from getting sick. Or, in other words, the vaccine efficacy tells us how much less likely a vaccinated person is to get sick compared to an unvaccinated person.
How can vaccines prevent the transmission of disease?
A vaccine teaches the immune system to recognize an infectious agent. If a person is later exposed to a virus like SARS-CoV-2, the immune system is already programmed with a response, helping to prevent infection or severe illness, according to the U.S. Food and Drug Administration (FDA), which oversees the approval of vaccines in the U.S. Vaccines protect a person's own immune system against contracting a pathogen or developing more serious forms of a disease once infected. This results in a less severe infection and lower viral load, which secondarily protects against transmission.
As Johns Hopkins Coronavirus Resource Center notes in its "Vaccines FAQ":
In general, most vaccines do not completely prevent infection but do prevent the infection from spreading within the body and from causing disease. Many vaccines can also prevent transmission, potentially leading to herd protection whereby unvaccinated people are protected from infection by the vaccinated people around them because they have less chance of exposure to the virus. We are still learning whether or not the current Covid-19 vaccines prevent transmission of SARS-CoV-2. It is likely they reduce the risk of virus transmission but probably not completely in everyone. This is one of the reasons why it will still be important for people to continue wearing masks and practicing physical distancing, even after being vaccinated.
Lead Stories contacted Pfizer for a statement but did not receive a response at the time of this writing. We will update the article as appropriate if they respond.