Is stiff person syndrome, the disease Celine Dion announced in December 2022 that she was diagnosed with, included in a list of adverse reactions reported after vaccination with the Comirnaty (Pfizer-BioNTech) mRNA COVID-19 vaccine? No, that's not true: Stiff person syndrome was included in the appendix of an April 2021 Pfizer report as part of a "list of adverse events of special interest." Adverse events are medically distinct from adverse reactions. As of this publication, stiff person syndrome has not been linked to the Pfizer COVID-19 vaccine.
The claim originated on Instagram on December 10, 2022. It showed a list of what it said were "adverse reactions" said to be associated with Pfizer-BioNTech Comirnaty vaccine, including stiff person syndrome. Text with the post read:
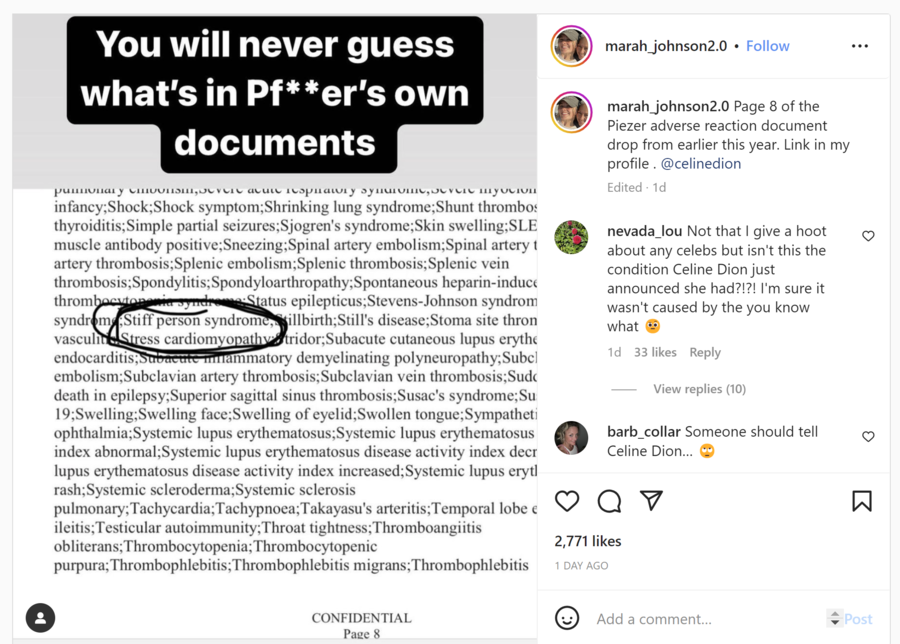
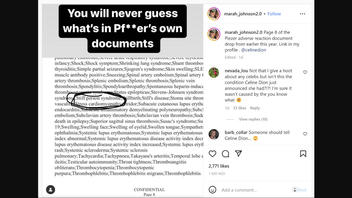
Page 8 of the Piezer adverse reaction document drop from earlier this year. Link in my profile . @celinedion
This is how the post appeared at the time of writing:
(Source: Instagram screenshot taken on Sun Dec 11 13:02:00 UTC 2022)
Inclusion in this list does not mean that stiff person syndrome had been observed following COVID vaccine administration, but rather that health care providers should report such an occurrence -- should it happen. The list shown in the Instagram post was taken from an April 2021 report published by Pfizer titled, "Cumulative Analysis of Post-authorization Adverse Event Reports." Stiff person syndrome, or Moersch-Woltman syndrome, was included on page 37 of the appendix in a "list of adverse events of special interest."
Adverse events are defined as unexpected medical problems -- including symptoms or disease -- that happen during treatment with a drug or therapy. These often occur in clinical settings or trials, as well as soon after a drug is put on the market. Uncommon and rare adverse events associated with COVID mRNA vaccines include anaphylaxis or myocarditis.
Adverse reactions (also called side effects) are medically different than adverse events. Reactions refer to known symptoms that may follow a vaccine, such as a fever or headache, whereas events describe previously unknown or unexpected medical conditions that may occur after vaccination. Adverse reactions and adverse events are not interchangeable medical terms despite often being confused with one another.
An adverse reaction is defined as known undesired effects that occur when a medication is given regardless of dosage. Common adverse reactions associated with COVID mRNA vaccines include fever, fatigue and pain at the vaccine injection site.
There are two types of adverse events. Type A adverse events are predictable and dose-related outcomes that are often identified in preclinical or clinical trials. Less common are Type B adverse events, which show up as unpredictable "bizarre or novel" responses to a particular drug or therapy often observed shortly after the drug has been put on the market. Type B adverse events can be influenced by a person's own vulnerabilities, such as allergies or intolerances.
Stiff person syndrome is not listed as an adverse reaction in package inserts included in Comirnaty vaccines or in fact sheets issued by both the U.S. Food and Drug Administration (FDA) and Centers for Disease Control and Prevention (CDC).
Lead Stories contacted Pfizer and the FDA for further comment but has not yet received a response. We will update the article accordingly.
Additional Lead Stories fact checks of claims about COVID-19 vaccines can be found here.