Was "vitamin" B17 prohibited for its "cancer-killing properties"? No, that's not true: The only thing it is known for is its ability to cause cyanide poisoning, not to treat cancer. Also, what social media refers to as a "vitamin" is not a vitamin.
The claim appeared in a video published on Facebook on March 1, 2023. A male narrator began:
The reason why people don't know what B17 is 'cause it's a vitamin. They were banned by the U.S. because of its cancer-killing properties.
The text appearing on the video contained a number of hashtags:
#holistichealth #B17 #vitamins #healthylifestyle #health #cancerprevention
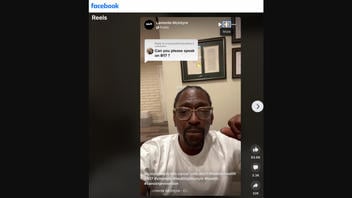
This is what the post looked like on Facebook at the time of writing:
(Source: Facebook screenshot taken on Tue Mar 7 15:58:17 2023 UTC)
The claim is not factual. "B17" is not a vitamin.
This is an alternative way to refer to amygdalin (also known as laetrile), which can be found in some seeds, nuts or kernels. The natural origin does not mean the product has any benefits or is safe to use.
A 1981 article published in the Cancer Journal For Clinicians called out its promoters for misleading consumers:
In 1970, Ernst Krebs, Jr. announced that he had discovered the etiology of all forms of cancer. Cancer, he concluded, was a vitamin deficiency disease, every bit as much as is pernicious anemia. According to Krebs, Jr., the missing vitamin in cancer was laetrile, which he called Vitamin B17 (he and his father had already invented Vitamin B15).
This so-called discovery accomplished several purposes. He no longer had to defend his earlier theory of laetrile's mechanism of action, which had been widely exposed as scientific nonsense. Moreover, if laetrile was now to be considered a vitamin, perhaps it would not be subjected to the more exacting federal legislation concerning the marketing of drugs. Finally, Krebs could capitalize on the American public's well-known love affair with vitamins.
There is no evidence that laetrile, or amygdalin, has any anti-cancerous properties.
In the 1980s, the National Cancer Institute conducted a study that found nothing to back up this theory:
Most of the patients in this study had breast, colon, or lung cancer. In about half of the patients, cancer had grown by the end of the treatment. Cancer had grown in all patients 7 months after treatment ended.
A 2015 review found no evidence that it may be effective at preventing cancer or killing cancer cells.
The Food and Drug Administration (FDA) has never approved amygdalin for treating not only cancer but any other medical condition. In 2017, the agency additionally listed it among the products promoted and sold "on websites or social media" consumers should be warned about:
They have not been reviewed by FDA for safety and efficacy, and can be dangerous to both people and pets.
Unlike unproven benefits, the risks of taking amygdalin are real. The FDA emphasizes that nature produces not only beneficial substances but also harmful ones:
The chemical is not found in the fruit itself and accidentally eating a seed or pit will not harm you. However, consuming a large amount of the seeds or pits can be problematic because enzymes in your intestines can turn amygdalin into cyanide and cause cyanide poisoning.
A 2017 paper described a case of a cancer patient who started developing multiple life-threatening symptoms 45 minutes after taking her very first dose of the so-called vitamin B17 and who was only able to survive because of rapid antidote administration at the hospital.
The FDA prohibits (in the Medication section) importing "unorthodox 'cures' for such medical conditions as cancer." In 1979, the Supreme Court banned its interstate transit.
Additionally, the FDA actively goes after the entities that mislead audiences with false ads, claiming that amygdalin or B17 may treat cancer.
Medicare does not cover amygdalin.
Yet it does not appear to be explicitly prohibited from being manufactured in the United States.
Moreover, during the 1970s, at least 20 states had allowed the use of the substance for terminal patients. Some of them, including West Virginia, introduced a provision preventing hospitals from intervening with the person's intake of amygdalin if it was prescribed by a licensed physician and the patient signed a consent form acknowledging that the substance was not approved by the FDA and that no major association of medical professionals supported its use. At certain localities, these provisions lasted for decades. For example, Maryland repealed it only in 2022.
Lead Stories previously reported on amygdalin here.