Has Pfizer instructed male COVID-19 vaccine recipients to refrain from donating sperm, to wear condoms and/or be abstinent for at least 28 days after getting a shot? No, that's not true: A screenshot used as the basis of the claim was taken from a lengthy protocol that was designed at the start of a clinical trial, approved on April 17, 2020. The implication of the screenshot is that the vaccine carries reproductive risks. But the precautions written into the protocol are not unusual and were not derived from known risks of the vaccine, rather they were derived from standard cautions that can be found in many clinical trials.
The screenshot, labeled in red lettering "Pfizer shot study, pg 135" with additional underlining and highlighting for emphasis, was posted to Instagram by formerly practicing OB/GYN, self-described women's health expert, author, wellness speaker and anti-vaccine influencer, Dr. Christiane Northrup on April 23, 2021. That Instagram post is no longer visible. Screenshots of Northrup's post are now circulating on Facebook -- for example this post (archived here) published by "Move Along; Nothing to See Here" on April 25, 2021. The text within the screenshot reads:
drchristianenorthrup
Pfizer shot study, pg 135104. Appendix 4: Contraceptive Guidance
10.4.1. Male Participant Reproductive Inclusion Criteria
Male participants are eligible to participate if they agree to the following requirements during the intervention period and for at least 28 days after the last dose of study intervention, which corresponds to the time needed to eliminate reproductive safety risk of the study intervention(S):
- Refrain from donating sperm.
PLUS either:
- Be abstinent from heterosexual intercourse with a female of childbearing potential as their preferred and usual lifestyle (abstinent on a long-term and persistent basis) and agree to remain abstinent.
OR
- Must agree to use a male condom when engaging in any activity that allows for passage of ejaculate to another person.
- In addition to male condom use, a highly effective method of contraception may be considered in WOCBP partners of male participants (refer to the list of highly effective methods below in Section 10.4.4).
This is what the post looked like on Facebook at the time of writing:
(Source: Facebook screenshot taken on Mon Apr 26 22:54:41 2021 UTC)
The text found in this screenshot appears in Pfizer's April 17, 2020, approved application (archived here) for a three-phase clinical trial of a RNA vaccine against COVID-19. This document has been reproduced in several forms, the page numbering and overall length of the document is not always the same. Section 10.4. Appendix 4 shown in the screenshot appears on Page 123 of this document, not page 135 as indicated in the screenshot.
This screenshot is real, but has been presented in a misleading way. Northrup has been advancing a baseless theory that the vaccine is altering human DNA and that vaccinated people now pose a danger to unvaccinated people they come in contact with, especially sexual partners, claims that Lead Stories has debunked prior to the date of this fact check. In February, Lead Stories consulted with independent infectious disease physicians and the FDA who cited peer-reviewed research showing that mRNA doesn't cross into the cell nucleus and does not rewrite the patient's genetic code. Nor is there medical evidence to support Northrup's assertion, as Lead Stories found when researching a claim that vaccinated persons shed infectious virus. This screenshot appeared in a PowerPoint slide presentation Northrup presented at the "Health and Freedom Conference" in Tulsa, Oklahoma on April 16, 2020. The contraceptive guidance was part of the directions and expectations clinical trial participants agreed to at the start of the trial.
Northrup is now using these precautionary contraceptive criteria to support her claim that the vaccine is dangerous.
Experts with relevant and current expertise say Northrup is wrong.
The American College of Obstetricians and Gynecologists published a news release on February 4, 2021, "Medical Experts Continue to Assert that COVID Vaccines Do Not Impact Fertility" which said, in part:
As experts in reproductive health, we continue to recommend that the vaccine be available to pregnant individuals. We also assure patients that there is no evidence that the vaccine can lead to loss of fertility. While fertility was not specifically studied in the clinical trials of the vaccine, no loss of fertility has been reported among trial participants or among the millions who have received the vaccines since their authorization, and no signs of infertility appeared in animal studies. Loss of fertility is scientifically unlikely.
In a 2002 book sponsored by the National Health Council, "Informed Consent: A Guide to the Risks and Benefits of Volunteering for Clinical Trials" by Kenneth Getz and Deborah Borfitz, the pact between the participant and researcher is explained as a means to protect the integrity of the data:
Always follow the study protocol. Protocols are very carefully designed. Failure to comply with the protocol can endanger you and can make the results of the trial inaccurate. Study volunteers who are instructed not to drink any alcoholic beverages but do so anyway may alter important lab results. Volunteers who fail to take the study medication as instructed or ignore their promise to use birth control and become pregnant (not to mention the unknown harm done to the fetus), may invalidate the clinical trial results, not to mention putting themselves at risk. Researchers need to understand how a study medication is working, whether doses need to be adjusted and what side effects the study participants are experiencing.
TransCelerate is a non-profit organization whose collaborating members are biopharmaceutical research and development organizations. Pfizer is one of the members. There have been efforts to standardize the layout and structure of clinical trial documents to make them easier to write and navigate, called the Common Protocol Template (CPT) Initiative. In 2017 some changes were made to the standardized research documents. In this presentation: slide 17-- the general descriptions of what is found in section 10.4. Appendix 4 of the core template is presented. Because of this standardization effort, it is quite simple to discover other clinical trials with very similar protocols to the one Northrup claims is proof of the danger of COVID-19 vaccines.
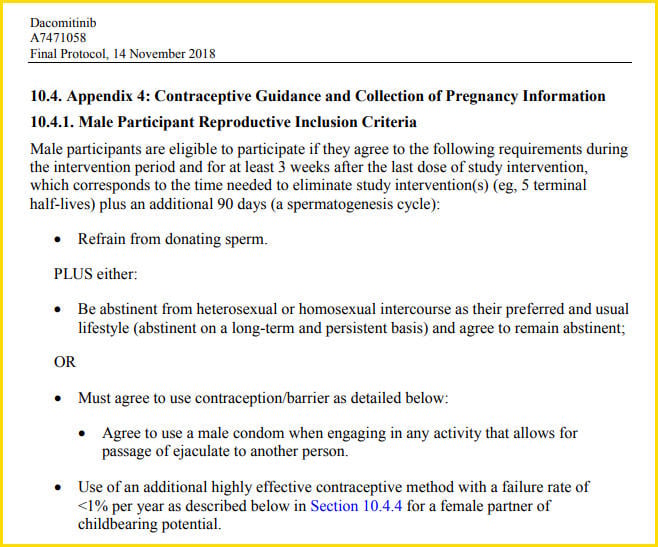
One example is a final Pfizer protocol for a non-COVID-19 drug, dated 14 November, 2018. This was a Hepatic Impairment Study, for a liver failure treatment. On Page 62 of the document the wording of section 10.4. Appendix 4 is almost identical to the Pfizer vaccine trial wording from Northrup's post -- with slightly longer wait time after the intervention period.
(Source: clinicaltrials.gov screenshot taken on Tue Apr 27 21:02:02 2021 UTC)
Another example can be seen in this October 31, 2018 final protocol. This study was a randomized double blind study to understand the pharmacokinetics (how a drug is absorbed, metabolized and eliminated) for a cream to treat atopic dermatitis.
(Source: clinicaltrials.gov screenshot taken on Tue Apr 27 21:14:02 2021 UTC)
A person with Dr. Northrup's expertise: a retired career medical professional who would have read scientific journals in medical school and while practicing, can be expected to understand and know that the wording of clinical trial protocols is standardized and based on a general abundance of caution as well as the design of each study -- not on specific known risks from a vaccine just starting a clinical trial.
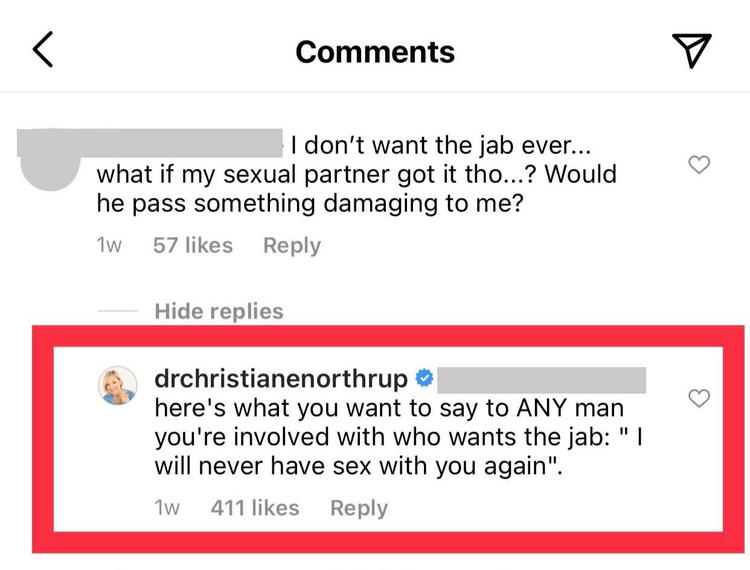
Lead Stories is not implying Northrup is circulating a baseless conspiracy theory to encourage her followers to pressure sexual partners not to get the vaccine. Below is documentation: a reply Northrup made to an Instagram follower who had just watched a Northrup video about women purportedly having unusual menstrual cycles after being near people who had been vaccinated.
(Source: Lead Stories highlighted Instagram screenshot taken on Mon Apr 26 23:46:02 2021 UTC)