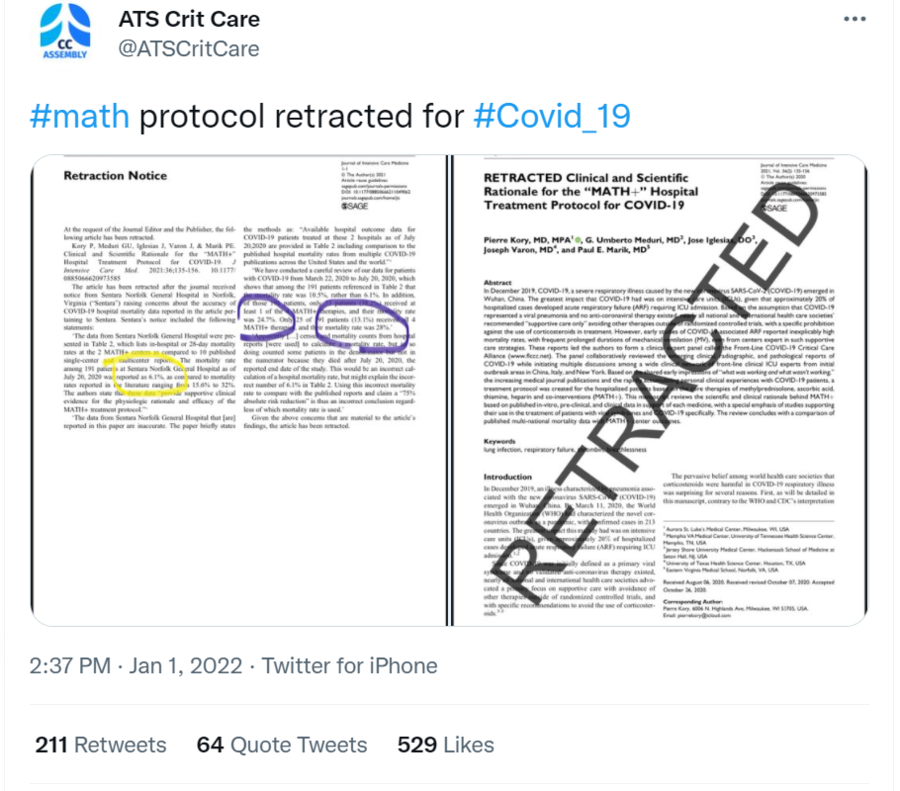
Was a study supporting the MATH+ treatment protocol for COVID-19 retracted after a hospital questioned the data used in it? Yes, that's true: The study backing the experimental and controversial regimen was pulled December 15, 2020, after the Journal of Intensive Care Medicine received notification from Sentara Norfolk General Hospital in Norfolk, Virginia, that raised concerns about the accuracy of the hospital's mortality data reported in the article and ultimately the study's conclusions. Instead of a mortality rate of 6.1% using MATH+, it was as high as 28% in some patient groups, the hospital said in statements printed with the retraction.
The claim appeared in a Twitter post (archived here) published by the American Thoracic Society Critical Care Assembly on January 1, 2022. It said:
"#math protocol retracted for #Covid_19"
This is what the post looked like on Twitter on January 4, 2022:
(Source: Twitter screenshot taken on Tue Jan 4 15:08:33 2022 UTC)
MATH+ is an experimental and controversial drug protocol consisting of blood thinners, steroids and vitamins. This is what the acronym stands for:
(Source: Graphic taken from study: MATH+ protocol for the treatment of SARS-CoV-2 infection: the scientific rationale)
The original study supporting MATH+ was published on August 18, 2020. The five authors -- Paul E. Marik, Pierre Kory, Joseph Varon, Jose Iglesias and G. Umberto Meduri -- are also physicians affiliated with the Front Line COVID-19 Critical Care Alliance (FLCCC), an anti-vaccine group that has been cited for perpetuating unsubstantiated claims regarding the virus in a scholarly article in Frontiers in Pharmacology. Marik, Kory, Varon and Iglesias are founding members of FLCCC.
In its retraction, the Journal of Intensive Care Medicine said the study was pulled at the request of the journal editor and the publisher. The notice appeared on December 15, 2020:
The article has been retracted after the journal received notice from Sentara Norfolk General Hospital in Norfolk, Virginia ("Sentara") raising concerns about the accuracy of COVID-19 hospital mortality data reported in the article pertaining to Sentara. Sentara's notice included the following statements:
"The data from Sentara Norfolk General Hospital were presented in Table 2, which lists in-hospital or 28-day mortality rates at the 2 MATH+ centers as compared to 10 published single-center and multicenter reports. The mortality rate among 191 patients at Sentara Norfolk General Hospital as of July 20, 2020 was reported as 6.1%, as compared to mortality rates reported in the literature ranging from 15.6% to 32%. The authors state that these data 'provide supportive clinical evidence for the physiologic rationale and efficacy of the MATH+ treatment protocol.'
"The data from Sentara Norfolk General Hospital that [are] reported in this paper are inaccurate. The paper briefly states the methods as: 'Available hospital outcome data for COVID-19 patients treated at these 2 hospitals as of July 20,2020 are provided in Table 2 including comparison to the published hospital mortality rates from multiple COVID-19 publications across the United States and the world.'
"We have conducted a careful review of our data for patients with COVID-19 from March 22, 2020 to July 20, 2020, which shows that among the 191 patients referenced in Table 2 that the mortality rate was 10.5%, rather than 6.1%. In addition, of those 191 patients, only 73 patients (38.2%) received at least 1 of the 4 MATH+ therapies, and their mortality rate was 24.7%. Only 25 of 191 patients (13.1%) received all 4 MATH+ therapies, and their mortality rate was 28%.
"Apparently [...] census and mortality counts from hospital reports [were used] to calculate a mortality rate, but in so doing counted some patients in the denominator but not in the numerator because they died after July 20, 2020, the reported end date of the study. This would be an incorrect calculation of a hospital mortality rate, but might explain the incorrect number of 6.1% in Table 2. Using this incorrect mortality rate to compare with the published reports and claim a '75% absolute risk reduction' is thus an incorrect conclusion regardless of which mortality rate is used."
Given the above concerns that are material to the article's findings, the article has been retracted.
The retraction was also published in the National Library of Medicine.