Is ivermectin recommended by the National Institutes of Health (NIH) or the U.S. government for the treatment of COVID-19 as of September 2022? No, that's not true: Neither the Centers for Disease Control and Prevention nor the Food and Drug Administration recommend the antiparasitic as treatment or prevention. As of this writing, the National Institutes of Health listed ivermectin as "being evaluated," as it has since 2020. As such, it is not true that ivermectin was suddenly listed on the NIH website.
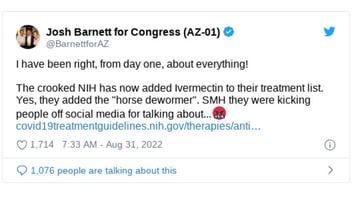
The claim appeared in an August 31, 2022, tweet by an Arizona congressional candidate. The tweet said: "the crooked NIH has now added Ivermectin to their treatment list."
This is what the post looked like on Twitter at the time of writing:
(Source: Twitter screenshot taken on Mon Sep 5 14:25:56 2022 UTC)
It is true that ivermectin is, in 2022, being studied as a potential treatment for COVID, so the drug is included on the NIH website as being evaluated. However, as of this writing, the antiparasitic drug has not been "approved" as a treatment or preventative for COVID in the use of humans. Nor is it true that ivermectin was suddenly listed on the NIH website in August, as the above social media post suggested. On the contrary, ivermectin has been tested as a possible treatment since 2020.
Ivermectin is typically used as an antiparasitic drug for animals, but it is also approved for the use of humans in certain instances. Ivermectin tablets, for example, are approved by the U.S. Food and Drug Administration (FDA) to treat people with two conditions caused by parasitic worms, strongyloidiasis and onchocerciasis. Topical forms can also be used to treat external parasites, such as lice, and for skin conditions like rosacea. The FDA, which is responsible for approving pharmaceuticals in the U.S., has not approved ivermectin for the treatment or prevention of COVID in either people or animals. (It should also be noted that ivermectin products for animals are different than those used for people.)
Since the onset of the pandemic, ivermectin has been at the source of mis- and disinformation campaigns focused on attacking the legitimacy of public health experts, as well as pushing false claims regarding safe and efficient treatments of and preventatives for COVID. To date, there has not been an emergency use authorization, recommendation, or approval for ivermectin in response to COVID.
The "ivermectin" section of the NIH landing page showed that the last update to the status of the drug occurred on April 29, 2022. At the time, the health agency noted that an expert panel recommended against the use of ivermectin for the treatment of COVID-19, except in clinical trials. (That status was current as of this writing.)
Health experts at NIH held that the use of ivermectin in the treatment of COVID has no "clinical benefit" at the time of this writing. Many studies, especially those that occurred early in the pandemic, had incomplete information or limitations in how they were conducted. Some of these studies, which had not been peer-reviewed, have since been retracted.
However, some studies have shown ivermectin may interfere with the virus's ability to infect humans and could hold possible anti-inflammatory properties that may be beneficial to people infected with the virus. But these are lab-based studies, not those conducted in humans, and would in some cases require the drug to be administered in doses up to 100-fold higher than those currently approved for use in people. Health experts maintain that "adequately powered, well-designed, and well-conducted trials are needed to evaluate the effect of ivermectin" on Covid. For more information on current clinical trials studying the effects of ivermectin, see the U.S. National Library of Medicine ClinicalTrials.gov.
Lead Stories contacted NIH seeking an update on recent scientific or clinical findings and where the drug stands in the approval process. We will update the story if we hear back.