Did the Food and Drug Administration (FDA) attempt to substitute "most salt in America" with a single alternative that contains "mRNA chemicals" and is produced by Microsoft co-founder Bill Gates? No, that's not true: An FDA representative told Lead Stories that the proposal would only expand the options for food manufacturers to use salt substitutes. The proposal, which is not yet a finalized rule, does not mandate such substitutions.
The story originated from an article (archived here) published by The People's Voice on August 26, 2023, under the title:
FDA Wants To Replace Salt With Bill Gates' New mRNA Fake Salt
It opened:
The FDA is quietly planning to replace most salt in America with a new synthetic 'salt substitute' produced by Bill Gates that will be laced with mRNA chemicals.
Here is what the claim looked like at the time of writing:
(Source: The People's Voice screenshot taken on Thu Aug 31 14:04:00 2023 UTC)
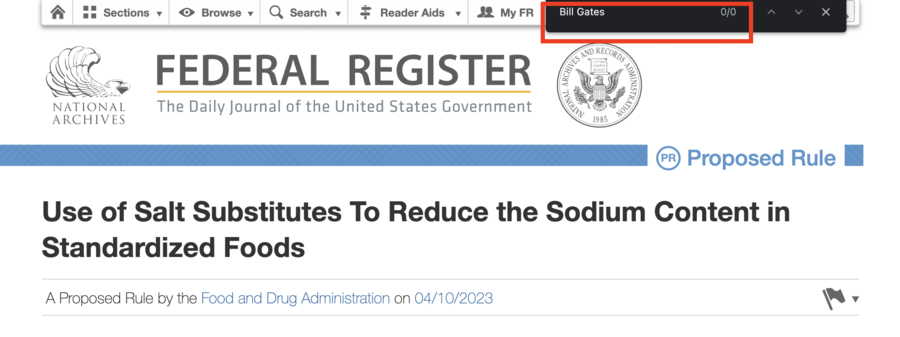
The second paragraph of the article cited a March 2023 PDF file from the FDA website titled "Pre-Publication of the Proposed Rule on the Use of Salt Substitutes to Reduce the Sodium Content in Standardized Foods." On April 10, 2023, this proposal was published in the Federal Register, the official record of changes in U.S. government regulations.
The proposal did not suggest replacing "most salt in America" with "Bill Gates' New mRNA Fake Salt" -- it did not even contain such words. See below the search for "mRNA" and for "Bill Gates":
(Source: Federal Register screenshot taken on Sep 1 20:42:41 2023 UTC)
(Source: Federal Register screenshot taken on Sep 1 20:43:14 2023 UTC)
The text of the proposal limited its impact to a specific category:
The Food and Drug Administration (FDA or we) is proposing to amend our standard of identity (SOI) regulations that specify salt (sodium chloride) as a required or optional ingredient to permit the use of salt substitutes in standardized foods, to reduce the sodium content.
The term "standardized food" refers to the products that have "standards of identity," or SOIs, which "often describe in detail what a food must contain and what is optional."
In an email to Lead Stories on September 1, 2023, an FDA spokesperson refuted The People's Voice claim about the proposal and clarified the nature of the document:
If this proposed rule is finalized, it would not require manufacturers to replace salt in standardized foods. Instead, by having the option to use a salt substitute, the rule provides greater flexibility when reformulating standardized foods to lower the sodium content. It would be up to the manufacturer to determine whether to make a substitution and how much of the sodium chloride (salt) could be substituted by safe and suitable ingredients. The extent to which salt can be replaced depends on the ability of salt substitutes to replace the functions of salt in the food without compromising the food safety or nutritional quality of the food.
The agency added:
Many foods do not have a standard of identity and currently have the flexibility to use salt substitutes.
The FDA spokesperson confirmed that the proposal has not yet been finalized as a rule. The period for public comments on the document ended on August 8, 2023, though, under government regulations, additional modifications can still be made until the rule is finalized.
No grounds exist to think the final rule will reflect the claim that is the target of this fact check. The People's Voice has a lengthy record of publishing false stories in the past. Its Facebook page, "The People's Voice," lost its verification checkmark, according to a 2018 report from Media Matters For America.
The website describes itself as a resource "comprised of various web pages operated by Fact Checked Limited," but it has nothing to do with fact checking.
As of this writing, it contained a liability disclaimer, saying:
FACT CHECKED LIMITED AND/OR ITS SUPPLIERS MAKE NO REPRESENTATIONS ABOUT THE SUITABILITY, RELIABILITY, AVAILABILITY, TIMELINESS, AND ACCURACY OF THE INFORMATION, SOFTWARE, PRODUCTS, SERVICES AND RELATED GRAPHICS CONTAINED ON THE SITE FOR ANY PURPOSE. TO THE MAXIMUM EXTENT PERMITTED BY APPLICABLE LAW, ALL SUCH INFORMATION, SOFTWARE, PRODUCTS, SERVICES AND RELATED GRAPHICS ARE PROVIDED 'AS IS' WITHOUT WARRANTY OR CONDITION OF ANY KIND.
Additional Lead Stories fact checks of claims about foods can be found here, here, here and here.