Are potential adverse reactions listed on a Hepatitis B vaccine package insert because the Food and Drug Administration says those adverse reactions and the vaccine are causally related? No, that's not true: The FDA requires manufacturers to include all potential adverse reactions reported during clinical trials or post-approval use, regardless of whether a causal relationship has been established. This is done to provide health care providers and patients with comprehensive information about the possible risks associated with the vaccine.
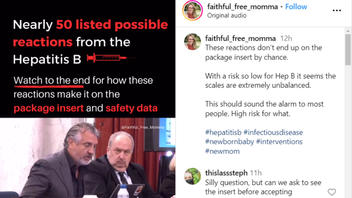
The claim appeared in a post and video (archived here) on Instagram by faithful_free_momma on March 4, 2024, under the on-screen title "Nearly 50 listed possible reactions from the Hepatitis B [vaccine]." The post's caption says:
These reactions don't end up on the package insert by chance.
This is what the post looked like on Instagram at the time of writing:
(Source: Instagram screenshot taken on Tue Mar 5 15:50:06 2024 UTC)
The video
During the 89-second clip, Del Bigtree, founder of the anti-vaccine group Informed Consent Action Network (ICAN), reads a list of about 50 reported adverse reactions or side effects from the package insert (archived here) for ENGERIX-B, a Hepatitis B vaccine made by GlaxoSmithKline Biologicals. When he completes the list about 45 seconds into the video, he adds:
That vaccine manufacturer has all these side effects in the warning list because why? it is stipulated by the FDA that there is a reasonable belief that they are causally related to the vaccine.
That's why they're there. They're not adding them just because they think they should, because they have to.
Food and Drug Administration
In a March 5, 2024, email to Lead Stories, the Food and Drug Administration (FDA) responded to the claim in the social media post. The agency's statement said:
The content within this video post does not provide an accurate description related to the safety of Engerix-B and is representative of the ongoing proliferation of misinformation and disinformation about the overall safety of vaccines which results in vaccine hesitancy and lowers vaccine uptake.
An adverse reaction is an undesirable effect, reasonably associated with use of a drug, that may occur as part of the pharmacological action of the drug or may be unpredictable in its occurrence. Federal regulations require a listing (within the product label/package insert) of adverse reactions that occur with the drug and with drugs in the same pharmacologically active and chemically related class, if applicable.
Separate lists are required for adverse reactions identified from clinical trials and those identified from spontaneous reports after a drug has been marketed.
Page 7 of the package insert for Engerix-B also explains why they're included. Ultimately, it's not because "there is a reasonable belief that they are causally related to the vaccine." Instead, it says:
The following adverse reactions have been identified during post-approval use of ENGERIX-B. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to the vaccine.
Bottom line: There is no proof of direct causation between the vaccine and the side effects, only that the reported adverse events followed the vaccine's clinical trials and FDA approval. Additionally, the package insert said:
The most common solicited adverse reactions were injection site soreness (22%) and fatigue (14%).
In its email to Lead Stories, the FDA added, "No SAEs [serious adverse events] were deemed related to Engerix-B" in 36 clinical studies, totaling 13,495 doses of Engerix-B that were administered to 5,071 healthy adults and children.
Read more
Additional Lead Stories fact checks of claims about vaccines can be found here.
Other Lead Stories fact checks of claims involving Bigtree can be found here.