Fact Check: Potential Adverse Reactions Are NOT Listed On Hepatitis B Vaccine Package Insert Because FDA Says They Are Causally Related
Fact Check
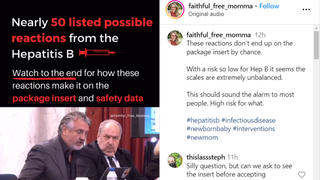
Are potential adverse reactions listed on a Hepatitis B vaccine package insert because the Food and Drug Administration says those adverse reactions and the vaccine are causally related? No, that's not true: The FDA requires manufacturers to include all potential adverse reactions reported during clinical trials or post-approval use, regardless of whether a causal relationship has been established. This is…