Did Physicians for Informed Consent correctly interpret the results of the drug trials by which the Janssen/J&J COVID-19 vaccine was approved and did that testing fail to establish whether the vaccine is effective and safer than COVID-19? No, that's not true: the Food and Drug Administration (FDA) found the Janssen vaccine was effective against COVID-19 and that the benefits of using it outweighed any harm. Several of the claims on the Physicians for Informed Consent website misstate the findings of the human tests of the vaccine.
The claim originates in a post (archived here) published by Physicians for Informed Consent on May 18, 2021 under the title "How effective is the Janssen (Johnson & Johnson) COVID-19 vaccine?" It opened:
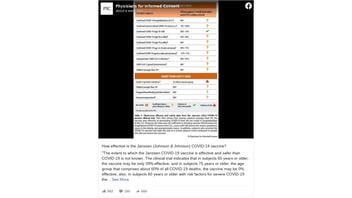
The extent to which the Janssen COVID-19 vaccine is effective and safer than COVID-19 is not known. The clinical trial indicates that in subjects 65 years or older, the vaccine may be only 39% effective, and in subjects 75 years or older, the age group that comprises about 60% of all COVID-19 deaths, the vaccine may be 0% effective.
This is what the post looked like on Facebook at the time of writing:
(Source: Facebook screenshot taken on Sat May 29 00:17:55 2021 UTC)
The primary claim -- that it is not known if the Janssen vaccine is effective -- is directly contradicted by the FDA's findings when it approved the Janssen injection as a treatment for fighting the COVID-19 pandemic.
In the 62-page report issued February 26, 2021, the FDA noted the drug was tested in human subject studies with 40,000 participants and concluded:
... based on the totality of scientific evidence available, the benefits of the Ad26.COV2.S vaccine outweigh its risks for
active immunization to prevent COVID-19 caused by SARS-CoV-2 in individuals 18 years of age and older.
The FDA also concluded:
The known benefits among recipients of the proposed vaccine relative to placebo are: Reduction in the risk of confirmed COVID-19 occurring at least 14 days after vaccination; Reduction in the risk of confirmed severe COVID-19 ... Efficacy findings were also generally consistent across evaluable subgroups, including by age, race, ethnicity, and risk for severe COVID-19.
A Janssen spokesperson, in a May 28, 2021, email to Lead Stories, said the vaccine-skeptical California group, Physicians for Informed Consent (PIC), misstated several details of the FDA's findings.
PIC: "The Janssen vaccine is only 39% effective for those 65 and older and It may be 0% effective for those over 75."
Janssen wrote in its email to Lead Stories that data published in the New England Journal of Medicine shows the effectiveness of the vaccine against symptomatic infections was similar among younger and older participants. Looking at Kaplan-Meier curves, an accepted method for estimating based on incomplete data, Janssen's spokesperson wrote that the data showed:
... cumulative incidence of cases among vaccine recipients 60 years of age or older with coexisting conditions was similar to that in the overall trial population, which suggests a similar vaccine efficacy. Vaccine efficacy against hospitalization among vaccine recipients 60 years of age or older with coexisting conditions was 82%, a finding consistent with this result.
PIC: "It's ineffective for those with severe risk conditions."
Janssen's spokesperson pointed out that the New England Journal of Medicine article includes data showing that, in the U.S., the effectiveness of the vaccine for moderate to severe-critical COVID-19 was 72% (58.2 to 81.7) at 28 days after vaccination. For severe-critical Covid-19, it was 85.9% (-9.4 to 99.7). Those levels are not deemed "ineffective."
PIC: There is "not have enough statistical material to measure ability to prevent hospitalizations and deaths."
But, the New England Journal of Medicine article published almost a full month before PIC published its claims showed that there were no hospitalizations for cases that arose at least 28 days after vaccination, while there were 16 hospitalizations in the placebo group. There were five COVID deaths in the placebo group. There were none in the group of trial participants who were vaccinated. Janssen's spokesperson underlined that finding, saying "The reduction in the incidence of death and the high efficacy against hospitalization are expected to substantially reduce the effect of this disease on individual persons and dramatically decrease the burden on health care systems."
PIC: There is "For those 18-39 ... the clinical trial did not include enough subjects to be able to show that the vaccine is safer than the disease"
Nearly one month before PIC published its fact sheets, the Advisory Committee on Immunization Practices noted about two dozen clotting-related reactions among the first 8 million people who received the Janssen vaccine, encouraging continued surveillance of patients, but continued use of the drug consistent with the FDA's original finding that the benefits outweigh the risks.
The "About" page on PIC's Facebook page says it is: "...dedicated to all the parents and legal guardians in California who lost their parental rights on June 30, 2015, when SB277 was signed into law and removed the personal belief and religious exemptions to childhood vaccination for both private and public school attendances."