STORY UPDATED: check for updates below.
Did Pfizer employees in Australia receive a special batch of imported COVID-19 vaccines that were different from what the public was given? No, that's not true: Pfizer global media relations told Lead Stories that the pharmaceutical and biotechnology company imported "additional doses" for its staff that were evaluated and authorized by Australia's Therapeutic Goods Administration (TGA) but all the COVID vaccines used in Australia were made at the same manufacturing plant.
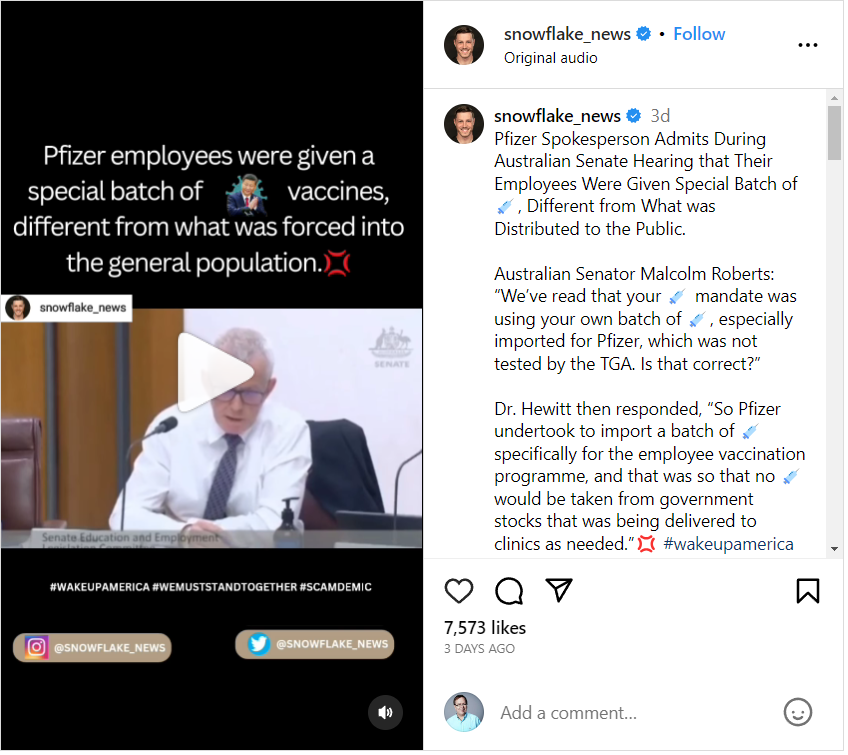
The claim appeared in a post and video on Instagram published by snowflake_news on August 5, 2023, under the title "Pfizer employees were given a special batch of vaccines, different from what was forced into the general population." The caption for the post said:
Pfizer Spokesperson Admits During Australian Senate Hearing that Their Employees Were Given Special Batch of 💉, Different from What was Distributed to the Public.
Australian Senator Malcolm Roberts: 'We've read that your 💉 mandate was using your own batch of 💉, especially imported for Pfizer, which was not tested by the TGA [Therapeutic Goods Administration]. Is that correct?'
Dr. [Brian] Hewitt [of Pfizer] then responded, 'So Pfizer undertook to import a batch of 💉 specifically for the employee vaccination programme, and that was so that no 💉 would be taken from government stocks that was being delivered to clinics as needed.'💢 #wakeupamerica #wakeupworld #wemuststandtogether ##freedom ...
#Australian #Australia #News #Headlines #BreakingNews #CurrentEvents #WorldNews #LocalNews #Trending #Journalism #Media #Information
This is what the post looked like on Instagram at the time of writing:
(Source: Instagram screenshot taken on Tue Aug 8 16:29:49 2023 UTC)
Here's the transcript of the video exchange included in the Instagram post:
Sen. Malcolm Roberts: We've read that your vaccine mandate was using your own batch of vaccine especially imported for Pfizer, which was not tested by the TGA. Is that correct?
Brian Hewitt: Senator, so, Pfizer undertook to import a batch of vaccines specifically for the employee vaccination program.
The video
The clip comes from an August 3, 2023, hearing of the Australian Parliament's Senate Education and Employment Committee. In a separate video posted to YouTube by Sen. Malcolm Roberts, he's seen questioning Brian Hewitt, head of regulatory sciences for Pfizer Australia and New Zealand, and Dr. Krishan Thiru, medical director for Pfizer Australia and New Zealand. Hewitt is answering the question posed by Roberts in the clip:
Pfizer
In an August 8, 2023, email to Lead Stories, Roma Nair, director of Pfizer global media relations, provided the company's response to the claim that Pfizer employees received different vaccines than the Australian public at large:
When Comirnaty became widely available in Australia, we wanted to offer Pfizer employees protection, but did not want to decrease the amount of vaccine available for the Australian public. As a result, we imported additional doses and made the vaccine available for staff. Pfizer employees received the vaccine at the same time as the general Australian population.
The Pfizer-BioNTech COVID-19 vaccine (Comirnaty) for Pfizer employees and for Australians was manufactured and supplied from Europe (Pfizer's one plant in Belgium). These doses were subject to evaluation and authorisation for supply by the Therapeutic Goods Administration (TGA) in accordance with the TGA's independent quality assessment of each batch of vaccine supplied in Australia.
Australia's TGA is responsible for evaluating, assessing and monitoring products that are defined as therapeutic goods. It regulates medicines, medical devices and biologicals. It's similar to the Food and Drug Administration in the United States.
Pfizer told Lead Stories that the batches used for the Pfizer Australian and New Zealand staff vaccination program were made public on the TGA website. Information regarding the TGA's batch release assessment of COVID-19 vaccines can be found here.
Australian Government Department of Health and Aged Care
The Australian Government Department of Health and Aged Care echoed Pfizer's response in an August 10, 2023, email to Lead Stories. A spokesperson for the department said:
All batches of Pfizer's Comirnaty vaccines and all other COVID-19 vaccines supplied in Australia, including those supplied directly to Pfizer employees, were required to comply with the quality and safety requirements of the Therapeutic Goods Administration (TGA). This includes post-market safety monitoring and vaccine batch release programs. The batches used for the Pfizer employee vaccination program were not different, separate or safer.
The batches for Pfizer employees were the same as those supplied to the Australian public in the Government vaccination program. The batches used in the Pfizer employee program were only available in small quantities and weren't suitable to meet the minimum delivery and packaging requirements for the public vaccination program. Rather than wasting the vaccines, Pfizer requested to use them as part of their own employee vaccination program.
All 7 batches used in Pfizer's employee vaccination program were reviewed through the TGA vaccine batch release process and tested by the European Official Control Authority Batch Release (OCABR) network of regulatory laboratories. ... There were 3 batches (FF0884, FE3064 and FC3558) used in Pfizer's employee vaccination program that were also distributed as part of the public vaccination program.
Additional Lead Stories fact checks of claims related to vaccines can be found here.
Updates:
-
2023-08-10T22:35:57Z 2023-08-10T22:35:57Z Adds response from the Australian Government Department of Health and Aged Care.