Did an RSV "vaccine" cause "12 deaths during clinical trials," as a post on Instagram suggested? No, that's not true: None of the 15 deaths of thousands of children in three key clinical trials were deemed to be caused by the drug. The Food and Drug Administration, AstraZeneca and an infectious disease expert all independently confirmed these results to Lead Stories. The post on Instagram appears to be referring to the RSV therapeutic Beyfortus, a monoclonal antibody that is not entirely the same as a traditional vaccine.
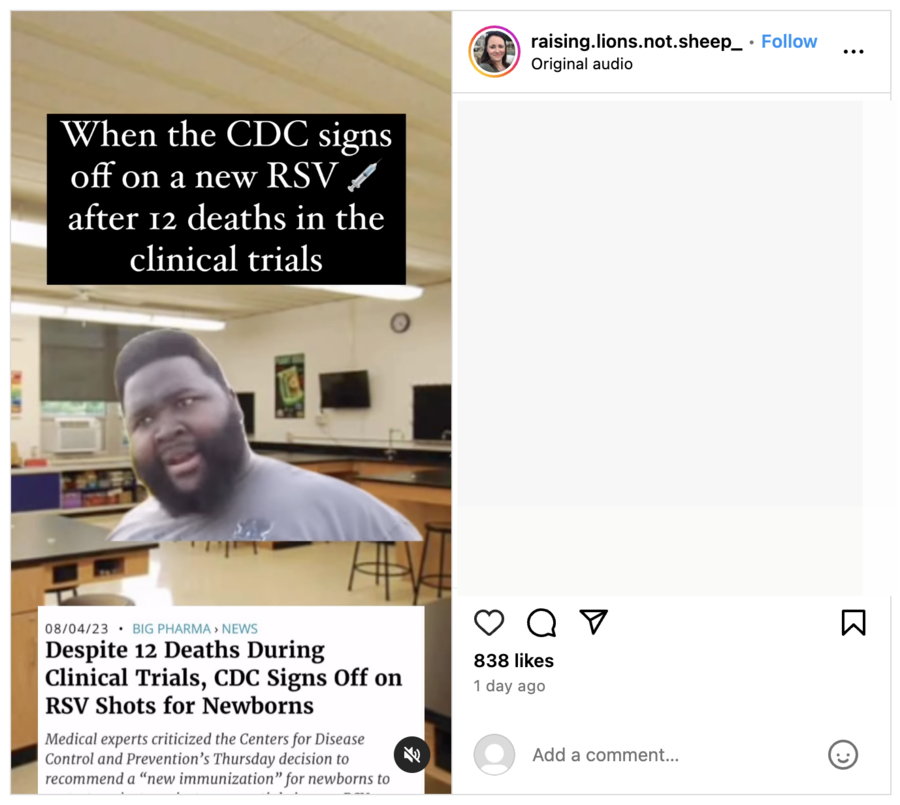
A version of the claim originated in a post on Instagram on January 27, 2024, (archived here) with a text overlay that read:
When the CDC signs off on a new RSV 💉 after 12 deaths in the clinical trials.
This is how the post appeared at the time of writing:
(Source: Instagram screenshot taken Mon Jan 29 09:59:58 UTC 2023)
This post included a screenshot of an article published by the Children's Health Defense on August 4, 2023, (archived here) titled, "Despite 12 Deaths During Clinical Trials, CDC Signs Off on RSV Shots for Newborns," which discussed clinical trials related to Beyfortus. An introduction to the article read:
Medical experts criticized the Centers for Disease Control and Prevention's Thursday decision to recommend a 'new immunization' for newborns to protect against respiratory syncytial virus, or RSV, calling the move unnecessary and not worth the known risks.
What is Beyfortus? What are monoclonal antibodies?
Beyfortus is a monoclonal antibody treatment given to prevent severe lung disease from RSV infection. It's described (archived here) as a "single dose long-acting" drug that contains the antibody nirsevimab.
Beyfortus and similar monoclonals are different than traditional vaccines in that they provide passive immunization, explained Dr. William Schaffner (archived here), a professor of infectious diseases at Vanderbilt University Medical Center in a phone interview on January 29, 2024.
The Food and Drug Administration (FDA) wrote Lead Stories in a January 30, 2024, email that "Monoclonal antibodies are laboratory-made proteins that mimic the immune system's ability to fight off harmful pathogens such as viruses. That is different from vaccines, which work by mimicking the infectious bacteria or viruses that cause disease. Vaccination stimulates the body's immune system to build up defenses against the infectious bacteria or virus (organism) without causing the disease."
Monoclonal antibodies provide "immediate protection" to the most vulnerable groups, like infants, Schaffner said. (More information on Beyfortus is provided in its packaging insert, archived here.)
What is RSV?
RSV, or respiratory syncytial, is described (archived here) by the Centers for Disease Control and Prevention (CDC) as a "common respiratory virus that usually causes mild, cold-like symptoms." Though most will recover in a week or two, infants who contract the virus are likely to develop severe infections and require hospitalization. It's estimated (archived here) that between 58,000 and 80,000 children under 5 years old in the U.S. are hospitalized each year due to RSV infection, with up to 300 deaths (archived here) in this age bracket.
Deaths in the study were not caused by Beyfortus, experts say
Many of the children died months after the drug was administered. Some had pre-existing conditions while another death was attributable to a car accident, drug manufacturer AstraZeneca confirmed.
"Beyfortus was given in the clinical trials not only to [healthy] infants but to infants at high risk. It would be expected that some of those infants, simply because they are high-risk infants, may have serious medical events that occur during the trial, including death," Schaffner said, adding that it's possible "the deaths would have happened anyway."
"Given the nature of these infants, the determination was made that Beyfortus was not causally related to the demise," he said. "Just because B follows A, doesn't mean that A -- the drug -- caused B, the deaths."
The FDA referred Lead Stories to this June 8, 2023, briefing document (archived here) compiled by AstraZeneca. It listed 15 deaths (pages 95-96), three of which were in the placebo group, 11 in the Beyfortus (Nirsevimab) group and one that received a different monoclonal antibody, palivizumab.
Lead Stories cross-referenced those same figures with trial results posted on ClinicalTrials.gov:
-
Trial 04 (archived here): Placebo deaths: 0, Beyfortus (MEDi18897): 4
-
Trial 05 (archived here): Palivizumab deaths: 1, Beyfortus (MEDi18897): 5
The FDA told Lead Stories by email:
Patient safety is paramount at the FDA and when the FDA grants a drug approval, it means the product has undergone a rigorous evaluation of safety, quality and effectiveness. The FDA carefully reviewed the cases of deaths reported in pivotal nirsevimab studies through 360 days post-dose to assess causality, and concluded that none of the deaths in the clinical trials were considered to be related to Beyfortus. The recorded deaths were attributed to underlying conditions such as congenital heart disease or other causes of infant mortality in the respective country or region where the trial subject was enrolled such as untreated gastroenteritis.
Beyfortus is manufactured by two companies, AstraZeneca and Sanofi, under a contractual agreement (archived here). In an email to Lead Stories received January 30, 2024, an AstraZeneca spokesperson confirmed the FDA's statement above, stating:
Every reported death was reviewed in detail for the cause of death, underlying co-morbidities, concurrently reported events and background rates in those populations. None of the deaths were considered related to Beyfortus by study investigators or by sponsors and these conclusions were aligned with the FDA's assessment. The causes of death were attributed to common causes of infant mortality reported in the region where the infant was enrolled, or to underlying medical conditions.
Further details regarding the deaths in each trial are provided on page 93 of the briefing document.
Beyfortus was approved in 2023 after three clinical trials
Beyfortus was approved by the FDA on July 17, 2023, for use in "neonates and infants born during or entering their first RSV season, and in children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season," according to a news release at the time (archived here).
On August 3, 2023, the CDC publicly recommended (archived here) Beyfortus after it had "been shown to reduce the risk of both hospitalizations and healthcare visits for RSV in infants by about 80 percent." The CDC suggests one dose for all infants younger than 8 months, as well as those born during or entering their first RSV season.
Beyfortus is also recommended by the American Academy of Pediatrics (archived here) and was voted in August 2023 (archived here) by the agency's Advisory Committee on Immunization Practices (ACIP) to include the drug in the Vaccines for Children program, which provides recommended vaccines and immunizations at no cost to about half the nation's children.
Read more
Additional Lead Stories fact checks of claims related to childhood vaccines can be found here.