STORY UPDATED: check for updates below.
Does a post that claims the World Health Organization now recommends against COVID-19 vaccination of children tell the whole story about the agency's recommendation? No, that's not true: WHO's June 3, 2021 guidance says more evidence is needed to make general recommendations. The public health agency's webpage on vaccination goes on to explain that children -- who tend to get less sick than adults -- are not the priority, unless they have certain health conditions that put them at higher risk if infected by the virus that causes COVID-19.
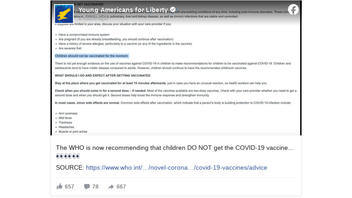
The post (archived here) was published on Facebook on June 22, 2021. It included what looked to be a screenshot of WHO's website, plus the following message:
The WHO is now recommending that children DO NOT get the COVID-19 vaccine...
This is what the post looked like at the time of writing:
(Source: Facebook screenshot taken on Tue Jun 22 19:40:33 2021 UTC)
WHO's Strategic Advisory Group of Experts (SAGE) has said that the Pfizer-BioNTech vaccine is suitable for children ages 12 and up. "Children aged between 12 and 15 who are at high risk may be offered this vaccine alongside other priority groups for vaccination. Vaccine trials for children are ongoing and WHO will update its recommendations when the evidence or epidemiological situation warrants a change in policy," says WHO.
The Facebook post links to this page on WHO's website. The page contains vaccination advice, including the following section on children (copied here in its entirety):
Children should not be vaccinated for the moment.
Children and adolescents tend to have milder disease compared to adults, so unless they are part of a group at higher risk of severe COVID-19, it is less urgent to vaccinate them than older people, those with chronic health conditions and health workers. More evidence is needed on the use of the different COVID-19 vaccines in children to be able to make general recommendations on vaccinating children against COVID-19. WHO's Strategic Advisory Group of Experts (SAGE) has concluded that the Pfizer/BionTech vaccine is suitable for use by people aged 12 years and above. Children aged between 12 and 15 who are at high risk may be offered this vaccine alongside other priority groups for vaccination. Vaccine trials for children are ongoing and WHO will update its recommendations when the evidence or epidemiological situation warrants a change in policy. It's important for children to continue to have the recommended childhood vaccines.
Children and adolescents tend to have milder disease compared to adults, so unless they are part of a group at higher risk of severe COVID-19, it is less urgent to vaccinate them than older people, those with chronic health conditions and health workers.
More evidence is needed on the use of the different COVID-19 vaccines in children to be able to make general recommendations on vaccinating children against COVID-19.
WHO's Strategic Advisory Group of Experts (SAGE) has concluded that the Pfizer/BionTech vaccine is suitable for use by people aged 12 years and above. Children aged between 12 and 15 who are at high risk may be offered this vaccine alongside other priority groups for vaccination. Vaccine trials for children are ongoing and WHO will update its recommendations when the evidence or epidemiological situation warrants a change in policy.
It's important for children to continue to have the recommended childhood vaccines.
Last month, Kate O'Brien, director of the Department of Immunization, Vaccines and Biologicals at WHO, shared an editorial in The Washington Post that was written by two pediatricians and vaccine researchers, who called for COVID-19 vaccines to go to those most vulnerable -- not healthy children.
#VaccinEquity is about prioritization of limited supplies and decisions how to maximize impact. https://t.co/MwsFrBWbN6
-- Kate OBrien (@Kate_L_OBrien) May 14, 2021
The universal vaccination of healthy children 2 to 11 years old simply shouldn't be a priority and may ultimately prove unnecessary. The relatively small group of children at risk because of underlying medical conditions should of course be offered the coronavirus vaccines, once they have been established as safe and effective for that age group. For that reason, the pediatric vaccine trials should be completed, so that immunization would be available for especially vulnerable young children. Otherwise, those tens of millions of vaccines can be put to much better use elsewhere in fighting the pandemic.
Updates:
-
2021-06-23T12:04:26Z 2021-06-23T12:04:26Z Updated with section from latest version of WHO page.